 |
 |
 |
| |
SWORD-1 & SWORD-2: Subgroup Analysis of 48-Week Results by Age, Race and Gender - Maintenance With DTG/RPV Effective Regardless of Age, Sex, or Race
|
| |
| |
Maintenance With DTG/RPV Effective Regardless of Age, Sex, or Race
IDWeek2017/IDSA, October 4-8, 2017, San Diego
Mark Mascolini
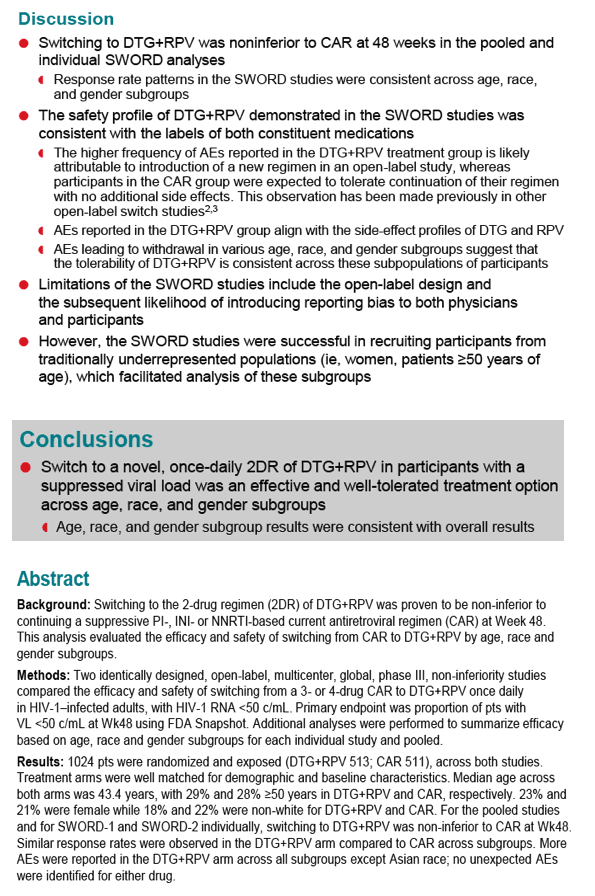
After 48 weeks in the combined SWORD 1 and 2 trials, similar proportions of virally suppressed people randomized to dolutegravir/rilpivirine (DTG/RPV) or a continued standard 3-drug regimen maintained a viral load below 50 copies, regardless of age, sex, or race [1]. But in most subgroups, adverse event rates proved higher with DTG/RPV than with a continued antiretroviral regimen.
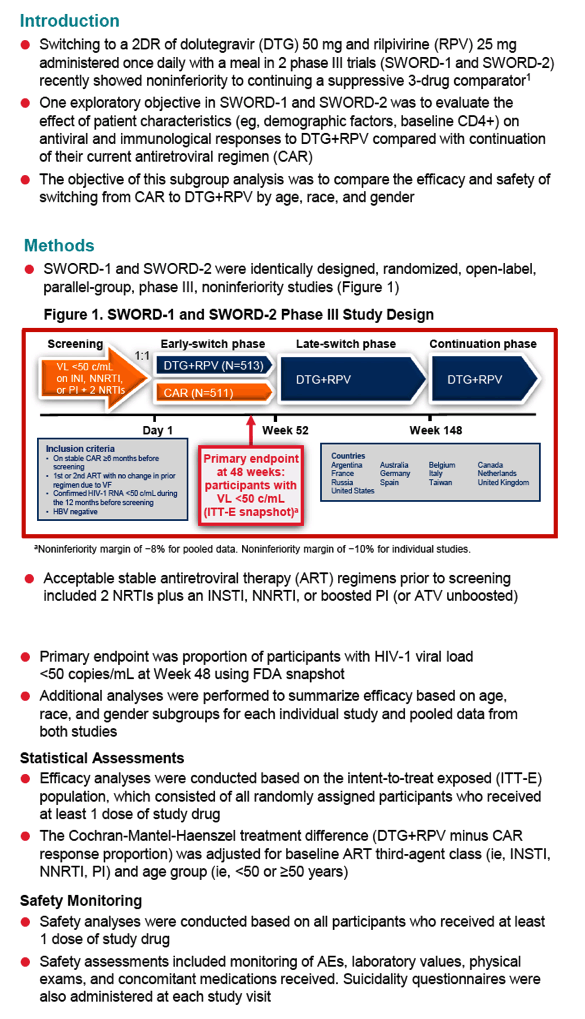
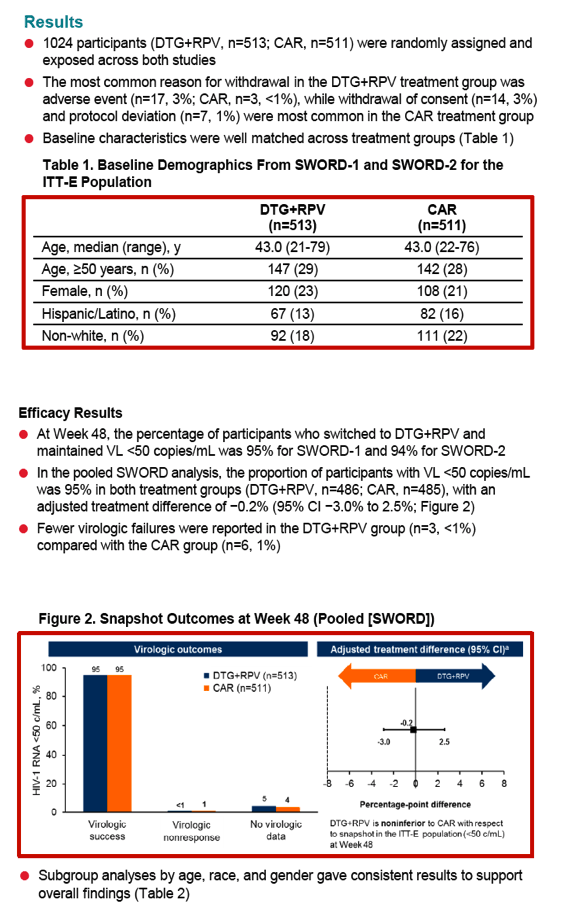
SWORD 1 and SWORD 2, identical international open-label trials, randomized people with a viral load below 50 copies for at least 12 months on a standard 3-drug regimen to stay with that combination or switch to once-daily DTG/RPV (50/25 mg). After 48 weeks 95% in both arms maintained a viral load below 50 copies by FDA snapshot analysis, a result establishing the noninferiority of switching to DTG/RPV [2]. There were fewer virologic failures with DTG/RPV than with standard therapy (3 versus 6).
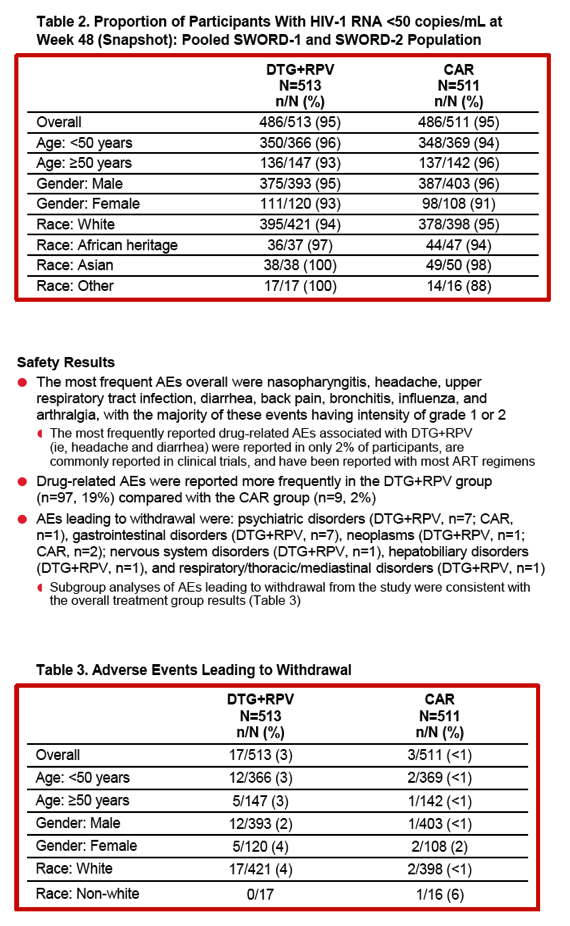
The new analysis compared 48-week results with DTG/RPV (n = 513) and standard therapy (n = 511) in three subgroups by age (under 50 or 50+), gender, or race (white, African, Asian, other). Demographic variables were similar in the two study arms (median age 43, about 29% older than 50, about 22% women, about 20% nonwhite). Sub-50-copy response rates proved nearly identical in the DTG/RPV arm and the control arm regardless of subgroup, except for a better response to DTG/RPV in "other" races:
-- Age under 50: 96% and 94%
-- Age 50 or older: 93% and 96%
-- Male: 95% and 96%
-- Female: 93% and 91%
-- White: 94% and 95%
-- Black: 97% and 94%
-- Asian: 100% and 98%
-- Other race: 100% and 88%
Drug-related adverse events proved more frequent with DTG/RPV than with continued standard therapy (19% versus 2%). Seven people assigned to DTG/RPV withdrew from the trial because of psychiatric disorders, as did 7 because of gastrointestinal complaints. Subgroup analysis found modestly but consistently higher adverse event rates with DTG/RPV than with a standard regimen except in nonwhites:
-- Age under 50: 3% versus under 1%
-- Age 50 or older: 3% versus under 1%
-- Male: 2% versus under 1%
-- Female: 4% versus 2%
-- White: 4% versus under 1%
-- Nonwhite: 0 of 17 versus 1 of 16 (6%)
The SWORD team observed that lower adverse event rates might be expected with a regimen already taken for 6 months or more than with a new regimen, especially in an open-label study. Of course a higher side-effect risk with a new regimen is one reason for staying with a suppressive tolerated regimen. The researchers added that adverse events seen with DTG/RPV are consistent with known side effect profiles of the two drugs.
References
1. Walmsley S, Richmond G, Bredeek F, et al. Sword 1 & 2: subgroup analysis of 48 week results by age, race and gender. IDWeek2017/IDSA. October 4-8, 2017. San Diego. Abstract 1382.
2. Llibre JM, Hung CC, Brinson C, et al. SWORD 1 & 2: Switch to DTG + RPV maintains virologic suppression through 48 weeks, a phase III study. Conference on Retroviruses and Opportunistic Infections (CROI). February 13-16, 2017, Seattle. Abstract 44LB. http://www.natap.org/2017/CROI/croi_11.htm
-------------------------
SWORD-1 & SWORD-2: Subgroup Analysis of 48-Week Results by Age, Race and Gender
Reported by Jules Levin
IDWeek2017/IDSA, October 4-8, 2017, San Diego
S Walmsley,1G Richmond,2F Bredeek,3M Ramgopal,4C-C Hung,5E Blair,6L Kahl,7M Underwood,6K Angelis,8K Vandermeulen,9B Wynne,10M Aboud7
1University Health Network, Toronto, Ontario, Canada; 2Holy Cross Hospital, Fort Lauderdale, FL; 3Maple Leaf Research, Toronto, Ontario, Canada; 4Midway Immunology and Research Center, Fort Pierce, FL; 5Department of Internal Medicine, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei,Taiwan; 6ViiV Healthcare, Research Triangle Park, NC; 7ViiV Healthcare, Brentford, UK; 8GlaxoSmithKline, Uxbridge, UK; 9Janssen, Beerse, Belgium; 10ViiV Healthcare, Collegeville, PA
|
| |
|
 |
 |
|
|