 |
 |
 |
| |
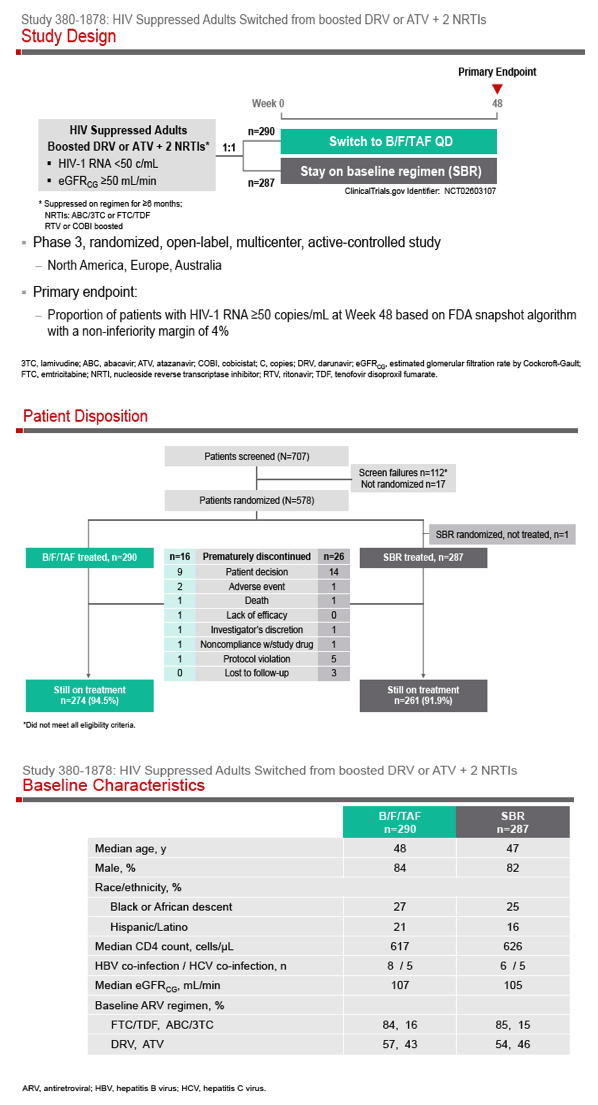
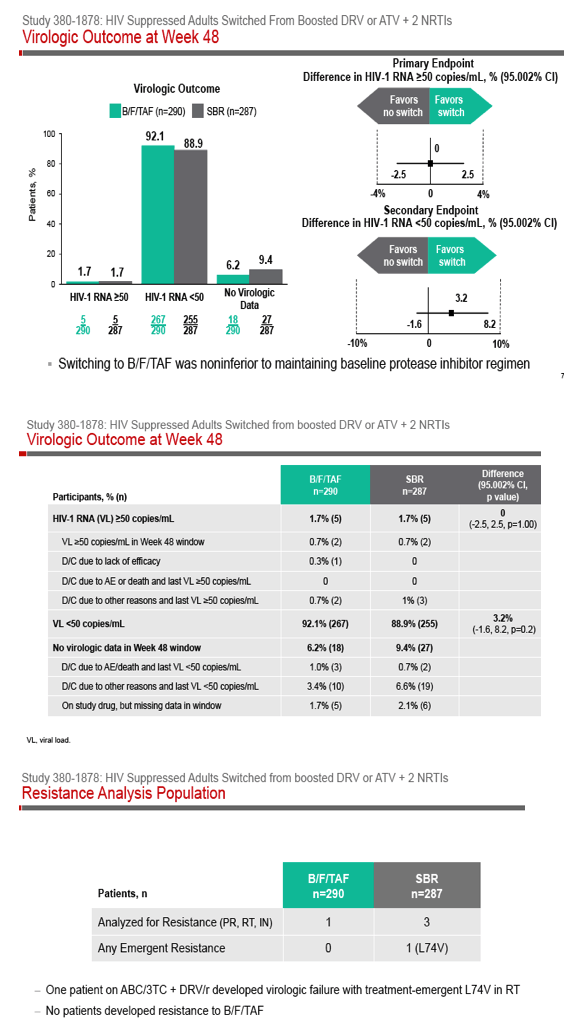
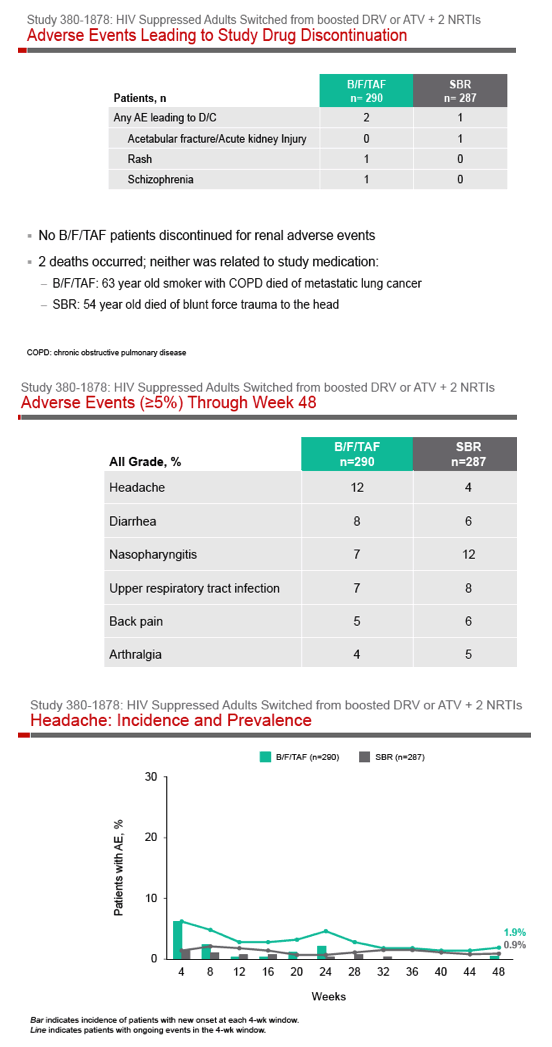
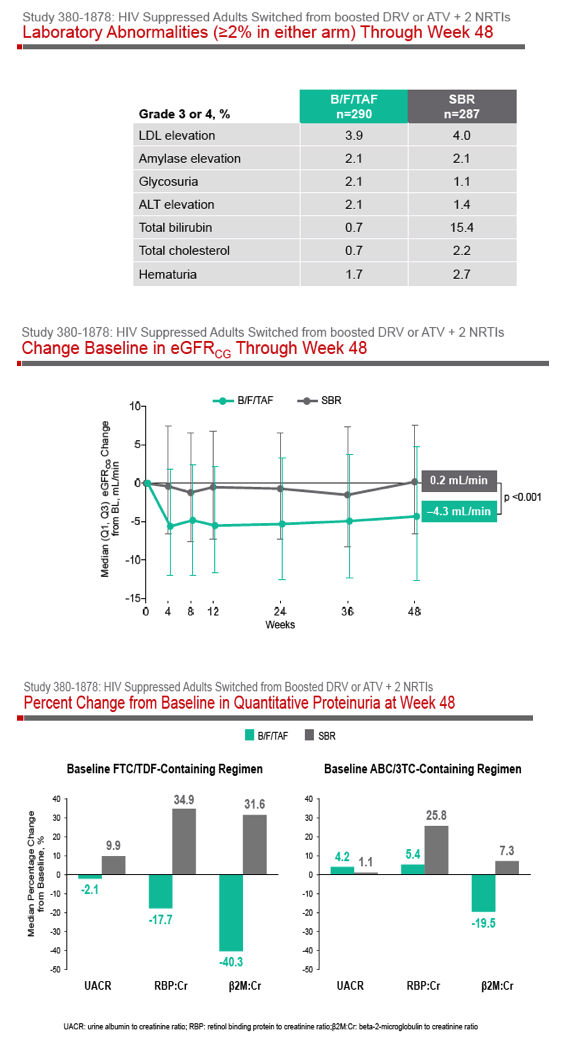
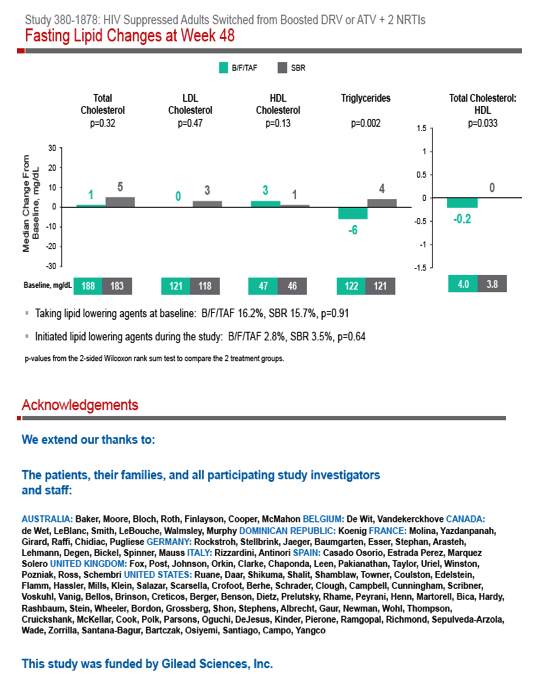
Phase 3 Randomized, Controlled Trial of Switching
to Fixed-Dose Bictegravir/Emtricitabine/Tenofovir
Alafenamide (B/F/TAF) from Boosted Protease
Inhibitor-Based Regimens in Virologically
Suppressed Adults: Week 48 Results
|
| |
| |
Reported by Jules Levin
ID Week 2017, October 4-8 San Diego, CA
Jurgen K Rockstroh,7 Jean-Michel Molina,8 Ellen Koenig,9 Ya-Pei Liu,10 Kristen Andreatta,10 Hiba Graham,10 Andrew Cheng,10 Hal Martin,10 and Erin Quirk10
(1) Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Torrance, CA, (2) Orlando Immunology Center, Orlando, FL, (3) Ruane Clinical
Research Group, Inc., Los Angeles, CA, (4) The Crofoot Research Center, Houston, TX, (5) Midland Florida Clinical Research Center, LLC, Deland, FL, (6) Howard Brown Health Center, Chicago, IL, (7) Department of Medicine, Bonn University Hospital, Venusburg, Germany, (8) Department of Infectious Diseases, Saint-Louis Hospital, Paris, France, (9) Medicine, Inst. Domin Estudio Virologicos, Santo Domingo, Dominican Republic, (10) Gilead Sciences, Foster City, CA

|
| |
|
 |
 |
|
|