 |
 |
 |
| |
The Effect of Hepatic or Renal Impairment on
Bictegravir Pharmacokinetics
|
| |
| |
Download the PDF here
Reported by Jules Levin
18th International Workshop on Clinical Pharmacology of Antiviral Therapy, June 14-16, 2017, Chicago, IL
Heather Zhang, Yongwu Shao, Will Garner, Amanda Vu, Hal Martin, Devi SenGupta, Erin Quirk, Brian P. Kearney, Joseph M. Custodio
Gilead Sciences, Inc., Foster City, California, USA
Gilead Submits New Drug Application to U.S. Food and Drug Administration for Fixed-Dose Combination of Bictegravir, Emtricitabine and Tenofovir Alafenamide for HIV Treatment - (06/13/17)
CROI: Randomized Trial of Bictegravir or Dolutegravir with FTC/TAF for Initial HIV Therapy - (02/16/17)
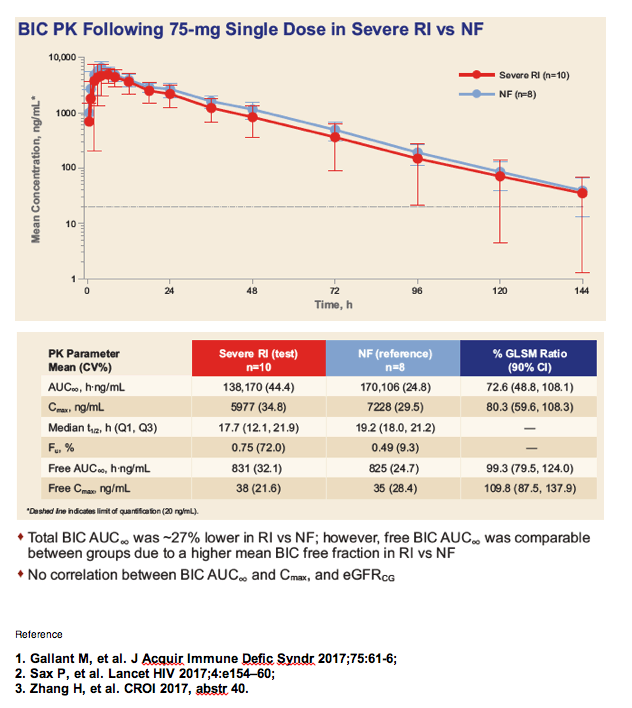
|
| |
|
 |
 |
|
|