 |
 |
 |
| |
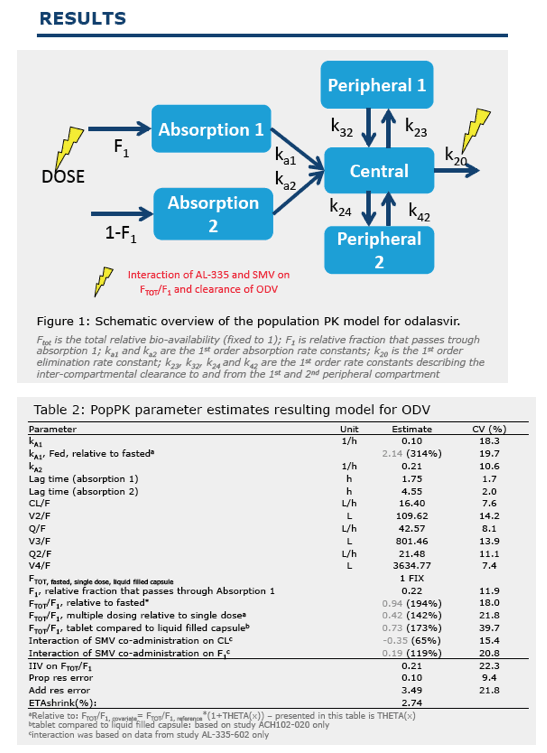
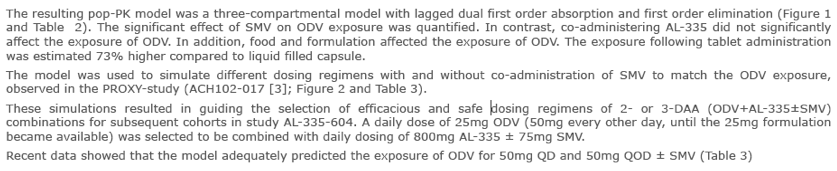
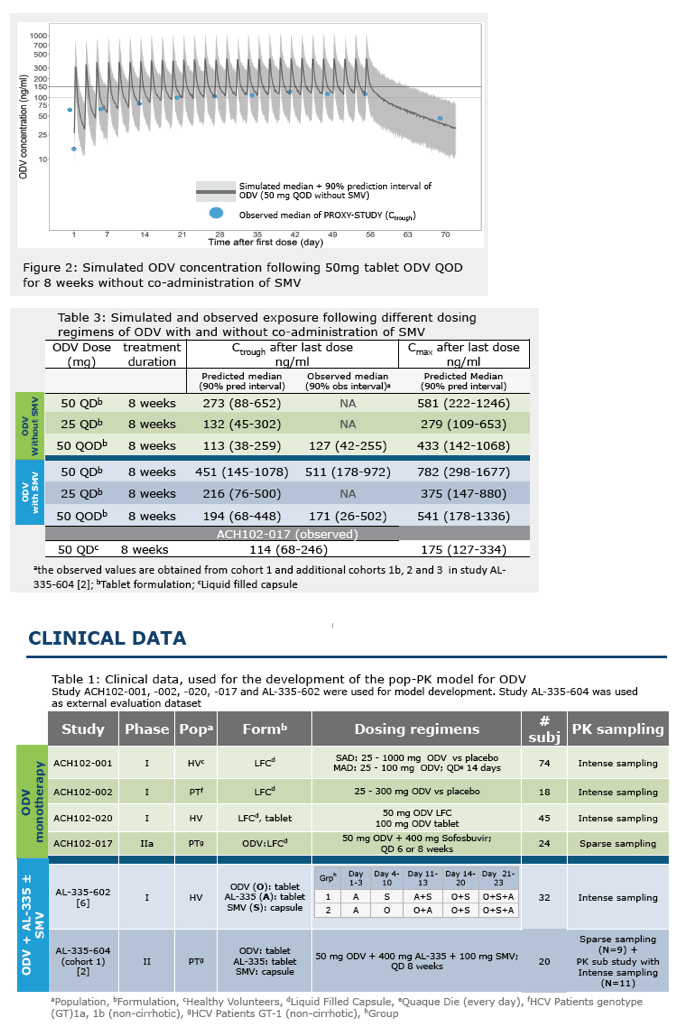
Dose-selection of odalasvir for use in combination with AL-335 and simeprevir in a phase 2A study using a population pharmacokinetic modelling and simulation approach
|
| |
| |
Reported by Jules Levin
18th International Workshop on Clinical Pharmacology of Antiviral Therapy, 14-16 June 2017, Chicago, IL,USA
Ackaert O1, Valade E1, Ouwerkerk-Mahadevan S1, Kakuda T2, Perez-Ruixo J1, Hoeben E1
1Global Clinical Pharmacology, Janssen R&D, Beerse, Belgium; 2Alios BioPharma, Inc., part of the Janssen Pharmaceutical Companies, South San Francisco, USA
Short Duration Treatment with AL-335 and Odalasvir, with or without Simeprevir, in Treatment Naïve Patients with Hepatitis C Infection with or without Cirrhosis - (04/24/17)
AL-335, A ONCE-DAILY PANGENOTYPIC NUCLEOTIDE HCV POLYMERASE INHIBITOR, DEMONSTRATES POTENT ANTIVIRAL ACTIVITY OVER 7 DAYS IN TREATMENT-NAïVE GENOTYPE 1-4 PATIENTS - (04/18/16)
PAN-GENOTYPIC EVALUATION OF AL-335, A CLINICAL STAGE URIDINE ANALOG INHIBITOR OF HEPATITIS C VIRUS POLYMERASE - (04/18/16)
Preclinical Characterization of AL-335, a Potent Uridine Based Nucleoside Polymerase Inhibitor for the Treatment of Chronic Hepatitis C - (05/05/15)



|
| |
|
 |
 |
|
|