 |
 |
 |
| |
Asymptomatic Neurocognitive Impairment With HIV Marked by Brain Atrophy
|
| |
| |
25th Conference on Retroviruses and Opportunistic Infections (CROI), March 4-7, 2018, Boston
Mark Mascolini
Compared with neuropsychologically normal men, those with HIV and asymptomatic neurocognitive impairment (ANI) had frontal white matter atrophy on brain imaging [1]. Longer HIV duration and lower CD4/CD8 ratio predicted brain atrophy.
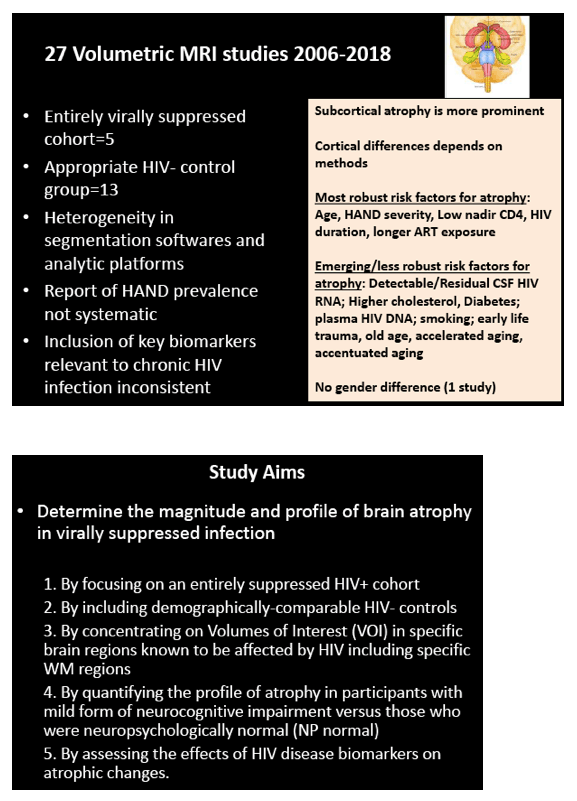
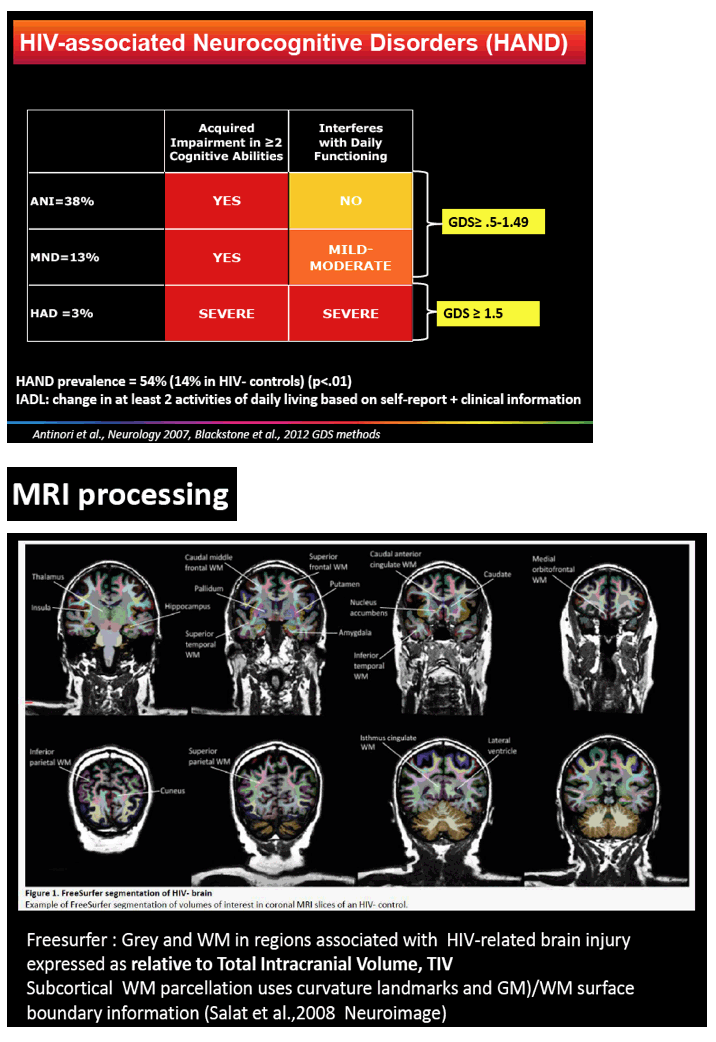
HIV-associated neurocognitive disorder (HAND), which persists in the current antiretroviral era, has three stages: ANI, mild neurocognitive disorder (MND), and HIV-associated dementia (HAD) [2]. Researchers in Sydney noted that controversy surrounds the meaning of ANI, which some argue is "a statistical neuropsychological entity with no neurobiological underpinning and no HIV causation." Lucette Cysique and colleagues hypothesized that people with well-controlled HIV infection and ANI would have HIV-related grey and white matter atrophy when compared with demographically similar HIV-negative controls.
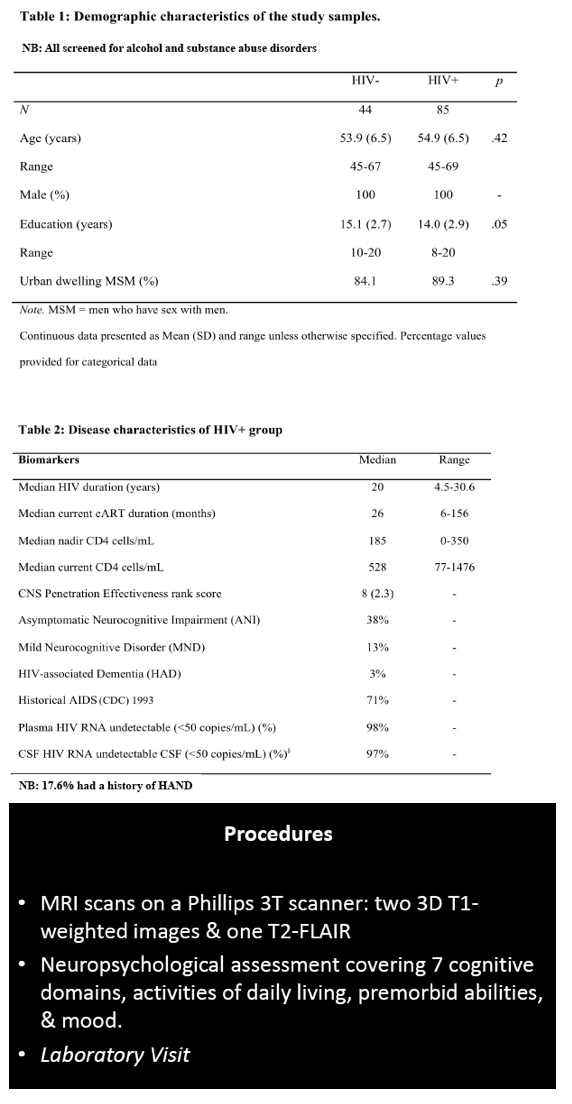
The study involved 85 men with HIV, almost all with a viral load below 50 copies in plasma and cerebrospinal fluid, and 44 demographically similar men without HIV. All underwent anatomical magnetic resonance imaging (MRI), neuropsychological evaluation, and HIV-related lab tests. The researchers used FreeSurfer software to extract grey and white matter regions linked to HIV-related brain injury.
Men with and without HIV were similar in average age (54.9 and 53.9) and urban residence (89.3% and 84.1%). Men with HIV had slightly fewer average years of education (14.0 versus 15.1, P = 0.05). The HIV group had been infected for a median of 20 years (range 4.5 to 30.6), had a nadir CD4 count of 185, and had a current CD4 count of 528. They had taken their current antiretroviral regimen for a median of 26 months (range 6 to 156). Among men with HIV, almost 1 in 5 (17.6%) had a history of HAND. When evaluated for this analysis, 38% had ANI, 13% MND, and 3% HAND; the rest were neuropsychologically normal.
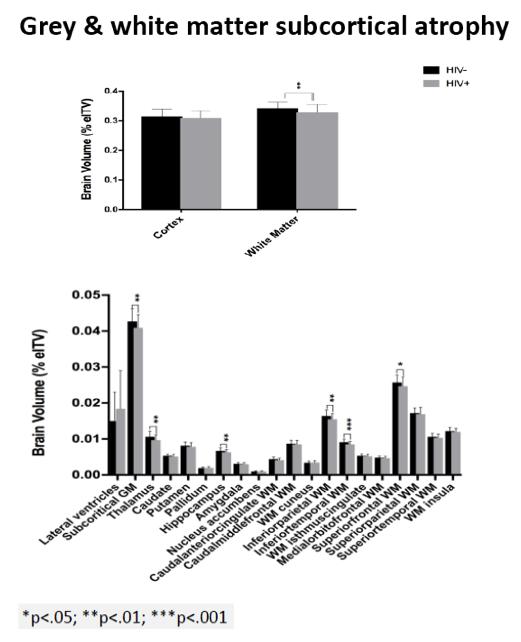
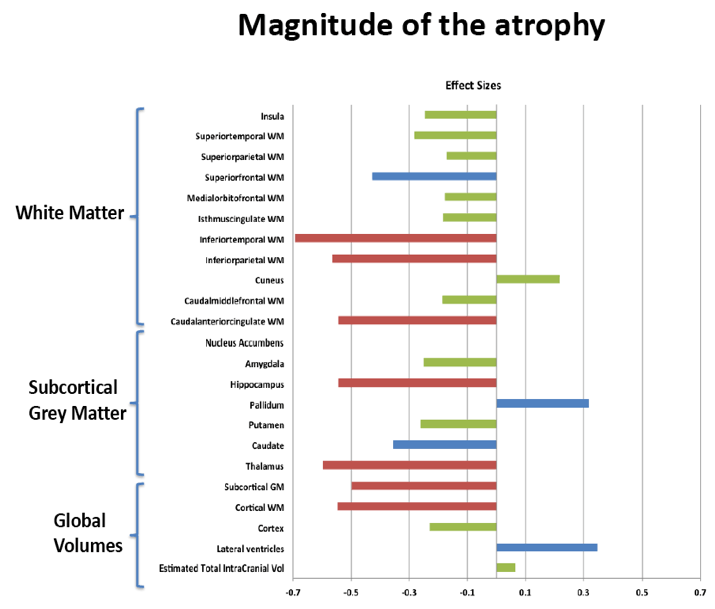
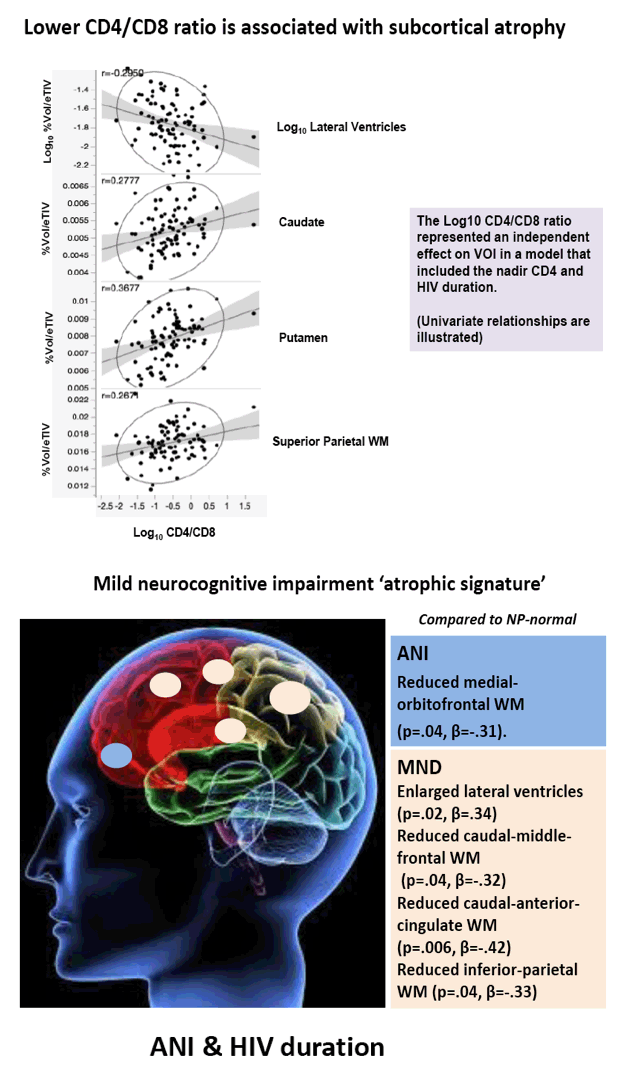
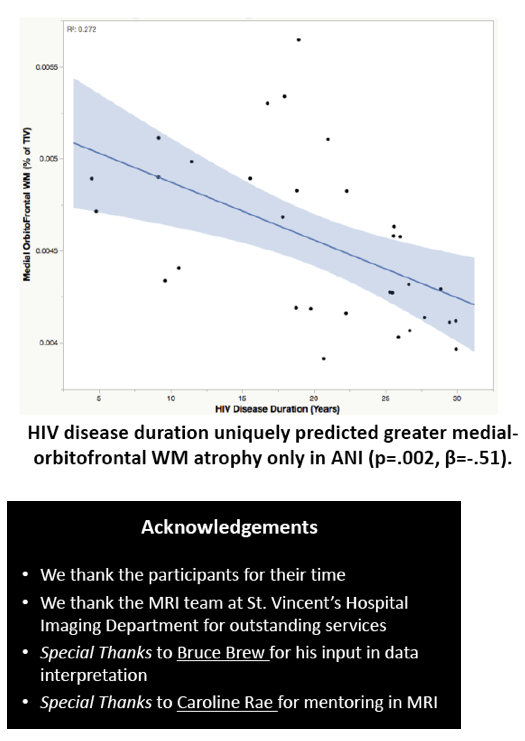
Compared with HIV-negative men, those with HIV had significantly lower white matter (d = 0.43 to 0.69, P < 0.01) and subcortical grey matter (d = 0.50 to 0.60, P < 0.01). Lower (worse) CD4/CD8 ratio was associated with subcortical atrophy. Compared with neuropsychologically normal men, those with ANI had significantly reduced medial-orbitofrontal white matter (beta = 0.31, P = 0.04). Among men with ANI, longer HIV disease duration predicted greater medial-orbitofrontal white matter atrophy (beta = 0.51, P = 0.002).
Compared with the neuropsychologically normal group, men with MND had enlarged lateral ventricles (beta = 0.34, P = 0.02), reduced caudal-middle-frontal white matter (beta = 0.32, P = 0.04), reduced caudal-anterior-cingulate white matter (beta = 0.42, P = 0.006), and reduced inferior-parietal white matter (beta = 0.33, P = 0.04).
The researchers concluded that ANI is linked to specific frontal white matter atrophy and that longer HIV duration is "a unique contributor to ANI-related brain atrophy." The Sydney team believes their findings "give neurobiological validity to ANI and may serve as an ANI biomarker."
References
1. Cysique L, Nichols M, Gates T, et al. HIV and brain atrophic signature in asymptomatic neurocognitive impairment. 25th Conference on Retroviruses and Opportunistic Infections (CROI). March 4-7, 2018. Boston. Abstract 121.
2. Clifford DB, Ances BM. HIV-associated neurocognitive disorder (HAND). Lancet Infect Dis. 2013;13:976-986. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4108270/
"ANI[asymptomatic neurologic impairment] is associated with specific frontal WM atrophy. HIV disease duration is a unique contributor to ANI related brain atrophy. These findings give neurobiological validity to ANI and may serve as an ANI biomarker."
WEBCAST: http://www.croiwebcasts.org/console/player/37287?mediaType=slideVideo&&crd_fl=1&ssmsrq=1520709667693&ctms=5000&csmsrq=1096

Program Abstract:
There is controversy as to whether Asymptomatic Neurocognitive Impairment (ANI) in HIV-Associated Neurocognitive Disorders (HAND) solely represents a statistical neuropsychological entity with no neurobiological underpinning and no HIV causation. We hypothesized that in a sample of non-confounded virally suppressed HIV+ persons versus demographically comparable controls, HIV-related grey and white matter (WM) atrophy could be observed in ANI.
85 HIV+ (plasma & CSF HIV RNA <50cp/mL, median nadir CD4=180, CD4=528) and 44 demographically comparable HIV- men (mean age=55; mean education=14.5 years; 90% men who have sex with men, 95% White Australian) underwent anatomical MRI, neuropsychological evaluation, and HIV laboratory tests. Volumes of interest (VOI) from MR images were extracted using Freesurfer to yield grey and WM in regions linked to HIV-related brain injury (total cortical volume, basal ganglia, lateral ventricles, fronto-striatal and fronto-parietal WM, all relative to Total Intracranial Volume, TIV). HAND status was ANI=38%, MND=13%, HAD= 3% based on the Global Deficit Score (GDS≥0.5) and functional decline; others were neuropsychologically (NP)-normal. A history of HAND occurred in 17.6%. We used multivariate analyses controlling for family-wise error rate to assess the effects of HIV status group on VOI. Next, ANI (N=32), MND (N=10) vs. NP-normal (N=40) and HIV biomarker (HIV duration, nadir CD4, current CD4 & CD8) effects on VOI were assessed with multiple regression controlling for a history of HAND.
Relative to the HIV- group, the HIV+ group demonstrated subcortical grey (d=0.50-0.60) and WM (d=0.43-0.69) atrophy, with relative cortical sparing (d=0.23). ANI showed reduced medial-orbitofrontal WM compared to NP-normal cases (p=.04, β=-.31). MND showed enlarged lateral ventricles (p=.02, β=.34), reduced caudal-middle-frontal WM (p=.04, β=-.32), reduced caudal-anterior-cingulate WM (p=.006, β=-.42), and reduced inferior-parietal WM (p=.04, β=-.33) compared to NP-normal cases. HIV disease duration predicted greater medial-orbitofrontal WM atrophy only in ANI (p=.002, β=-.51; Fig.1). Higher CD8 were independently associated with atrophy in the inferior-parietal WM (p=.003, β=-.41).
ANI is associated with specific frontal WM atrophy. HIV disease duration is a unique contributor to ANI related brain atrophy. These findings give neurobiological validity to ANI and may serve as an ANI biomarker.
|
| |
|
 |
 |
|
|