 |
 |
 |
| |
Durable inhibition of hepatitis B virusreplication and antigenemia using
subcutaneously administered siRNA agentAB-729 in preclinical models
|
| |
| |
Pdf of slide presentation attached so you can enlarge & read better
Reported by Jules Levin
EASL 2018 April 11-15 Paris France
Download the PDF Here
Amy C.H. Lee
Program Abstract:
Amy C.H Lee1, James Heyes1, Xin Ye1, Richard Holland1, Emily P. Thi1, Mark Wood1, Adam Judge1,
Nicholas M. Snead1, Alan Martin1, Michael J. Sofia2
1Arbutus Biopharma, Burnaby, Canada; 2Arbutus Biopharma, Warminster, United States
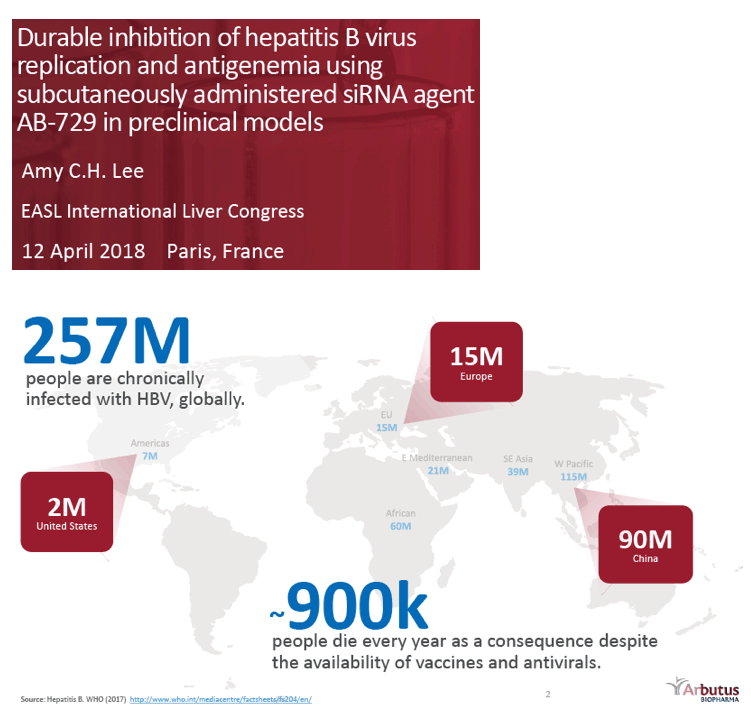
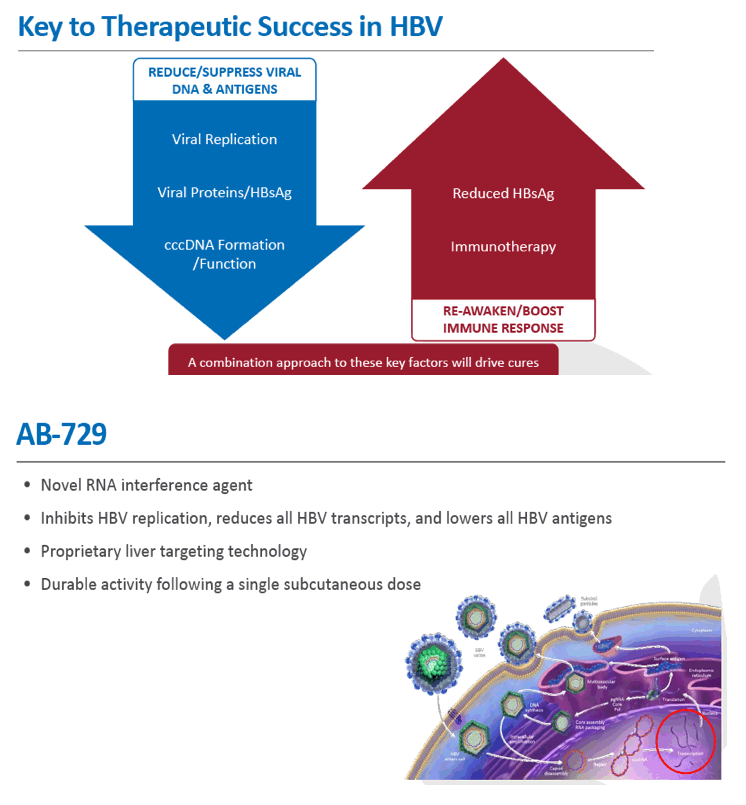
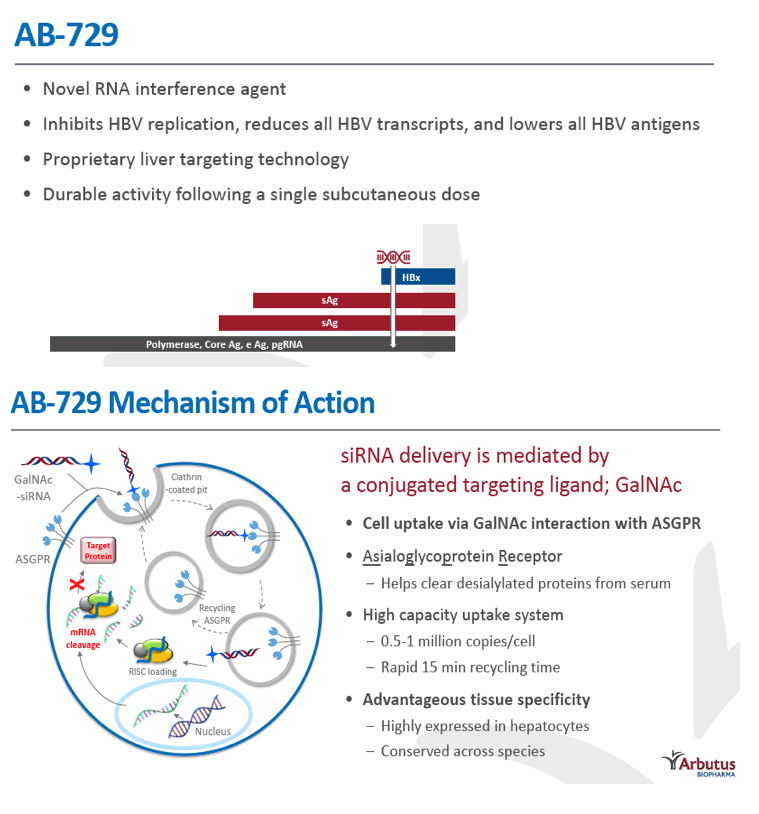
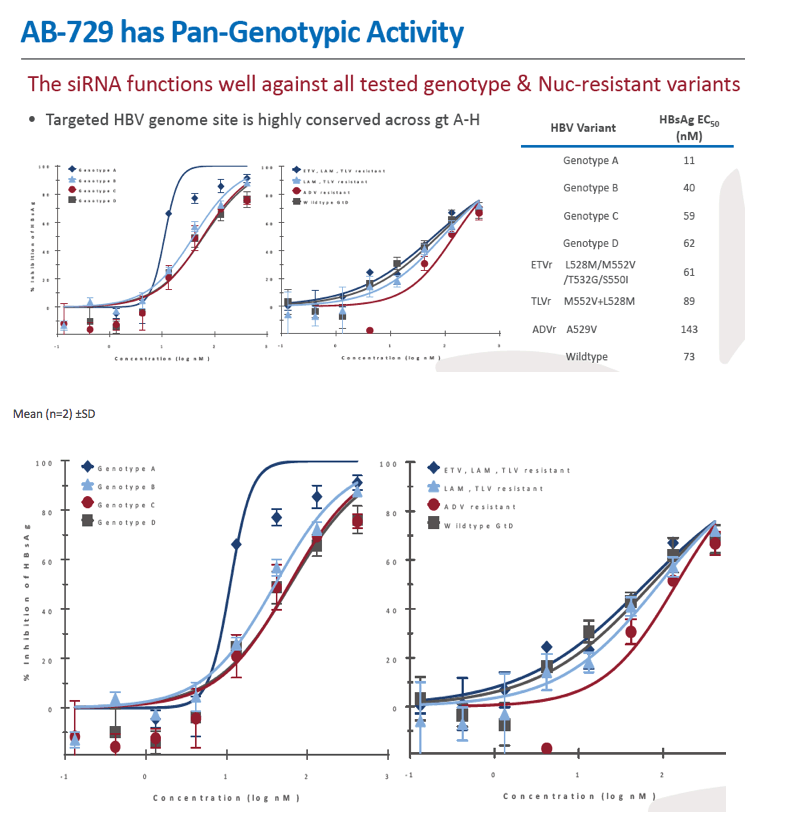
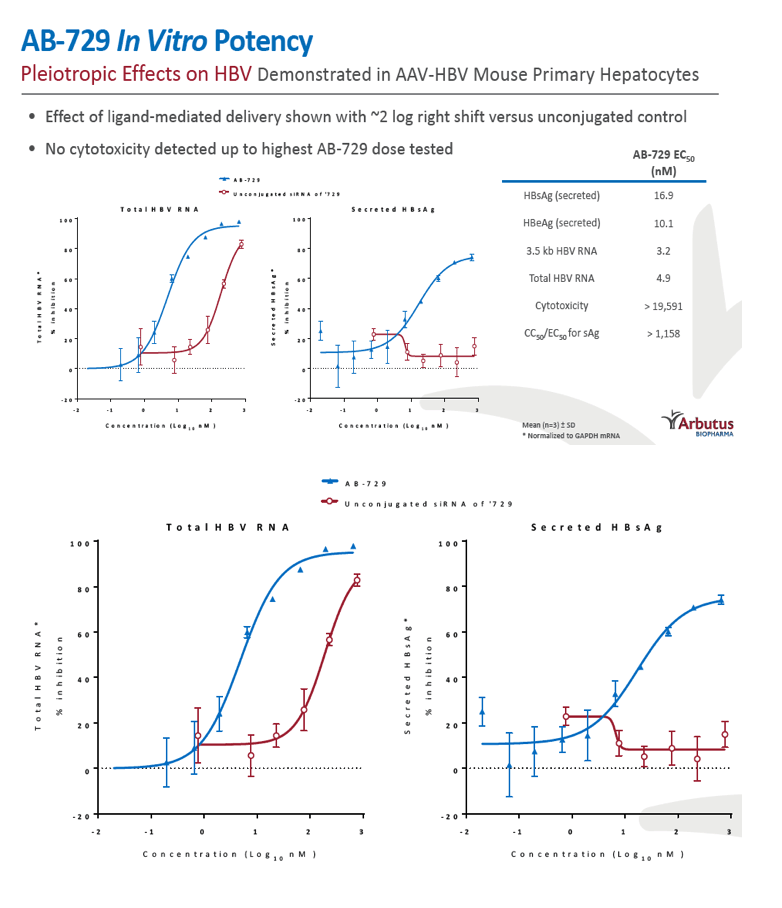
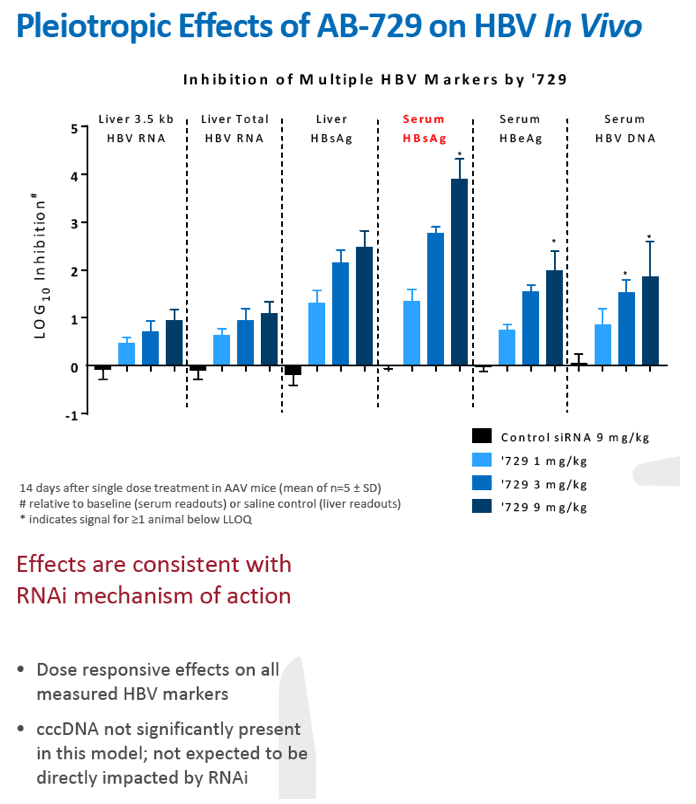
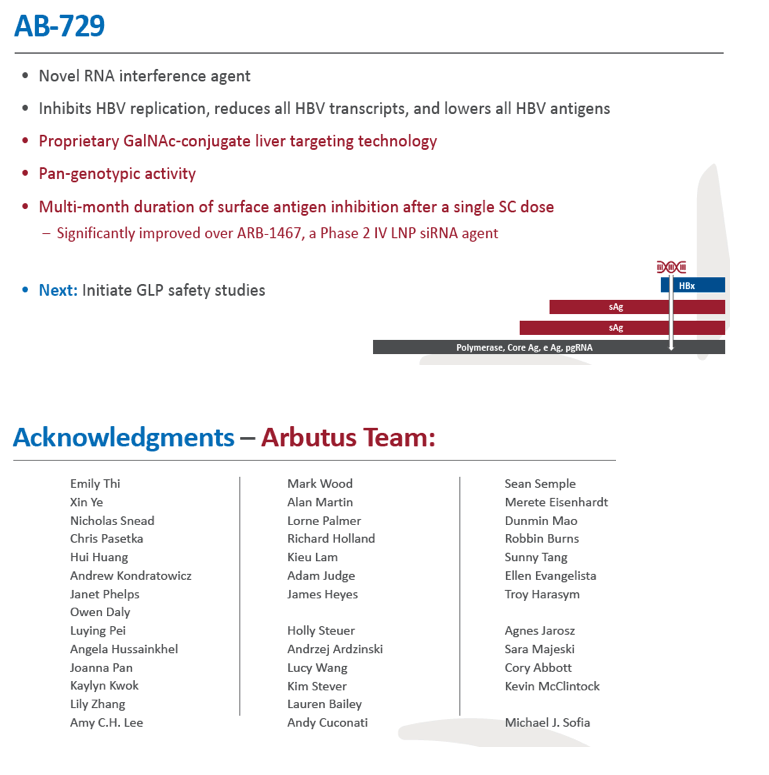
Background and Aims: Developing a cure for chronic hepatitis B (CHB) must address multiple factors involved in viral persistence and likely will require combination of drugs with different modes of action. Reducing HBV proteins, particularly HBsAg, may abrogate viral suppression of immune function and facilitate reinvigoration of host defense. We have recently reported up to 2.7 log HBsAg reduction from ARB-1467 treatment in CHB patients regardless of HBeAg status and with favorable safety profile (AASLD 2017). Here we describe ARB-270729, a next-generation small interfering RNA (siRNA) therapeutic targeted to hepatocytes using a novel covalently conjugated Nacetylgalactosamine (GalNAc) moiety. ARB-270729 is a promising new agent to potentiate HBV cure and acts on multiple hepatitis B viral transcripts, enabling inhibition of HBV replication and suppression of all viral antigens.
Method: In vitro anti-HBV activity was studied in primary and immortalized hepatocyte culture systems and in vivo anti-HBV activity was studied in a tolerized mouse model of CHB, C57BL/6 mice infected with an adenovirus-associated virus (AAV) carrying a 1.2-fold overlength genome of genotype D.
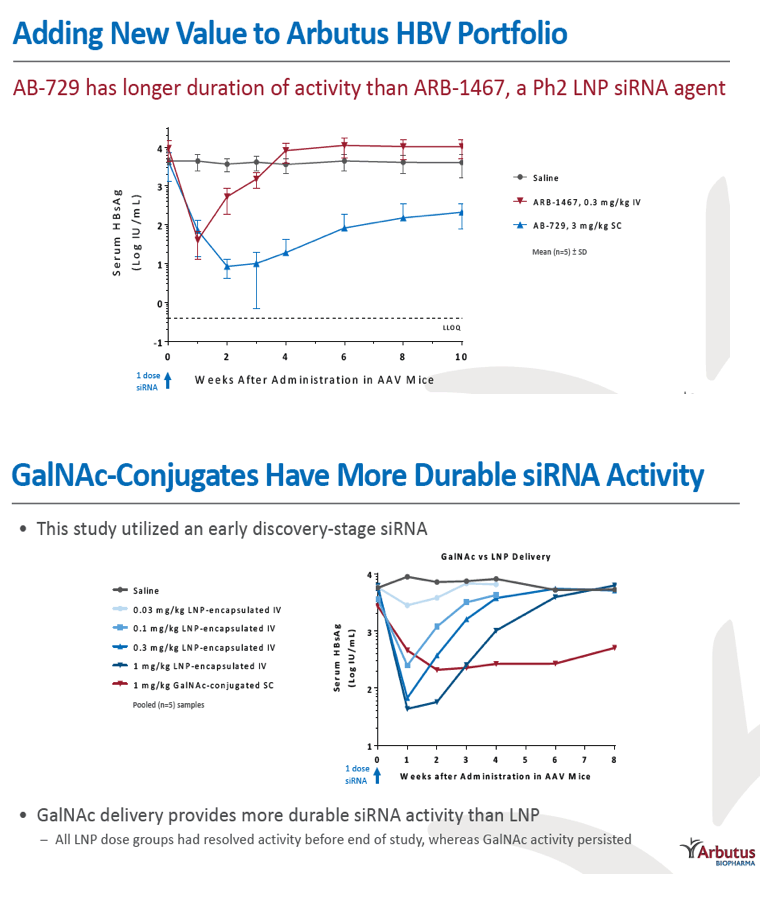
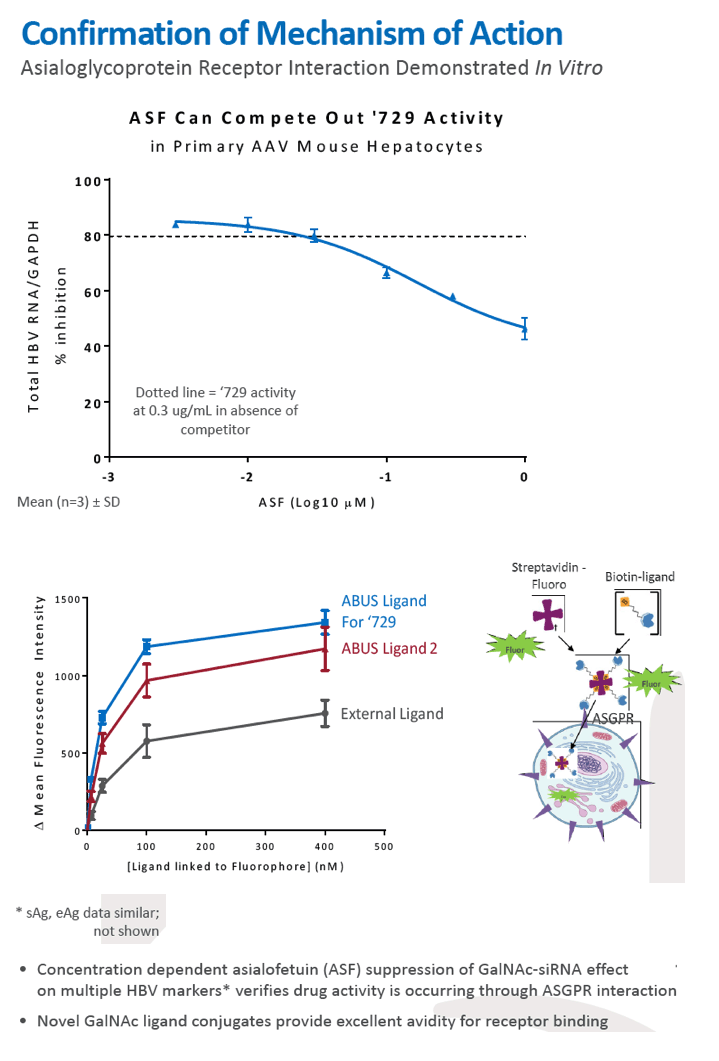
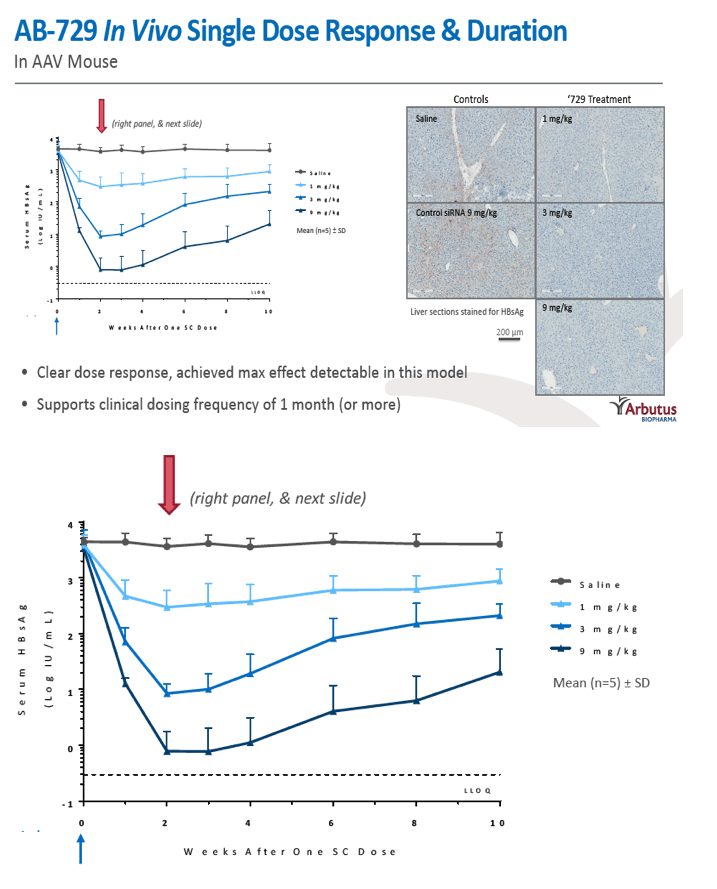
Results: In comparison to lipid nanoparticle (LNP)-mediated intravenous delivery, GalNAc-conjugated subcutaneous delivery of the same reference siRNA required a 10-fold larger dose to achieve similar mean maximum inhibition of serum surface antigen (HBsAg) in AAV-HBV mice. However, HBsAg suppression in the LNP treatment group had fully resolved by Week 4 whereas the GalNAc treatment group nadir persisted from Week 2 through to Week 6. One dose of ARB-270729 was sufficient to achieve mean maximum HBsAg reductions of 1.1, 2.4 and 3.5log10 at 1, 3 and 9mg/kg, respectively, in AAV-HBV mice with baseline serum HBsAg 3.6 log10IU/ml. In vivo ARB-270729 suppression of HBsAg was also highly durable, with 83%, 89% and 99%, respectively, of the mean maximal effect remaining at Week 10 after a single dose. The GalNAc moiety is required for ARB-270729 activity; reduction of HBV RNA and HBsAg was completely blocked by asialofetuin competitive inhibition in vitro.
Conclusion: ARB-270729 is a next-generation siRNA agent possessing a well-defined mechanism of action as well as a different route of administration and more durable in vivo preclinical activity than earlier-generation siRNA agents for the treatment of chronic hepatitis B infection.
|
| |
|
 |
 |
|
|