| |
|
HCV Test & Treat - Hepatitis C Virus Diagnosis and the Holy Grail
|
| |
| |
Download the PDF here
".....innovative funding mechanisms to drive program scale up are needed.
It is time the world took HCV diagnostics seriously. Wide-spread diagnosis of HCV is essential to achieve global elimination and increasing access is now a public health priority. The global community must come together to collectively support efforts to develop testing strategies that are patient-centric, expedite assay registration, reduce costs, and generate the demand required for businesses to invest in wide-scale roll out of existing products. No one diagnostic solution will fit all purposes, but the global community must invest in partnerships that facilitate the development and introduction of the holy grail POC HCV diagnostic test to ensure finding the missing millions in need of curative treatment."
"Although very few new classes of POC diagnostics have come to the market in recent years,117 the world can expect the arrival of transformational technologies in the next few years. Fundamental advances in each diagnostic assay component are underway, including new assay chemistries and nanotechnologies to improve sample capture and detection, and novel materials and microfluidics to allow miniaturization.118, 119, 120 Smart phone-assisted diagnosis of infection, through either the adaptation of reading devices or addition of biosensing platforms to a mobile device, are also on the horizon and promise to improve access to testing, including in rural and remote communities.121, 122 Investments to maximize smart phone diagnostic innovations may provide opportunities to improve health care more broadly, including improved surveillance data collection in remote and resource-limited settings, increased telehealth capacity, and the ability to more easily implement interventions to enhance linkage to care.123 The availability of new materials and innovative solutions such as 3-dimensional printing are also likely to further reduce costs and facilitate the scale-up of many of these technologies.124, 125 Although many of these new classes of diagnostic tools have been developed for other viral infections, such as HIV or Zika, these advances can undoubtedly be quickly translated to HCV diagnosis and management if there is commitment."
-----------------------
Hepatitis C Virus Diagnosis and the Holy Grail
Tanya L. Applegate, B.App Sc (Hons), PhDa,*, Emmanuel Fajardo, BSc (Hons), MScb,
Jilian A. Sacks, PhDc
The world has embraced the call for global elimination of hepatitis C virus by 2030. The unprecedented speed of therapeutic development and increased access to direct-acting antivirals has made elimination a possibility. We must screen hundreds of millions of people to diagnose and treat those currently infected. Global access to hepatitis C virus diagnostics will be a keystone to success. Key challenges must be overcome and systems optimized to ensure widespread access to existing diagnostics. Although promising technologies may soon transform the landscape, innovative strategies are needed to stimulate investment and accelerate the development of point-of-care hepatitis C virus diagnostics.
Key points
o Innovative strategies to provide access to existing HCV diagnostic assays are an immediate priority.
o Decentralized models of care to diagnose HCV infection and confirm cure within community health care settings are critical to achieve HCV elimination by 2030.
o High quality, simple, affordable and rapid diagnosis of active infection at the point-of-care will be central to achievement of HCV elimination.
o Global partnerships and funding mechanisms are required to stimulate investment in the development of the holy grail - the ideal point-of-care diagnostic test.
Hepatitis C virus diagnostics are essential to achieve global elimination
It is not often the world has the opportunity to turn a public health crisis into a good news story.1, 2 The development of oral, highly effective, pangenotypic direct-acting antivirals (DAAs) has now paved the way to cure the 71 million people estimated to be living with chronic hepatitis C virus (HCV) infection globally.3, 4, 5, 6 Unfortunately, fewer than 20% of those living with HCV are aware of their infection, and the challenge now is to engage, screen, and diagnose everyone in need of treatment.7, 8 While the world has focused its attention on the final steps within the cascade of care to develop and increase access to DAAs over the last decade,9, 10, 11 considerably less investment has been made to ensure accurate and affordable diagnostic tools10 are available to make wide-scale global treatment a reality (Fig. 1). Ironically, in many settings, prohibitively high costs of HCV diagnostics often now exceed the cost of curative therapy.12 Improving access to rapid, simple, and affordable HCV diagnostics is critical to achieve global HCV elimination.13, 14, 15
The high efficacy and low toxicity of the new DAAs provide an exceptional opportunity to greatly simplify HCV diagnosis and care. Recently approved pangenotypic DAA regimens no longer depend on quantitative HCV RNA or genotype data to stratify the duration of treatment,16, 17, 18 and many international clinical trials and demonstration studies are underway to generate evidence of the efficacy of a simplified approach to diagnosis and treatment monitoring (eg, in Cambodia, India, Iran, Rwanda, Nigeria, Mozambique, Myanmar, Pakistan, and Uzbekistan19). Likewise, excellent safety profiles, potent efficacy, and limited potential drug-drug interactions also negate the need for intensive on-treatment monitoring.20, 21
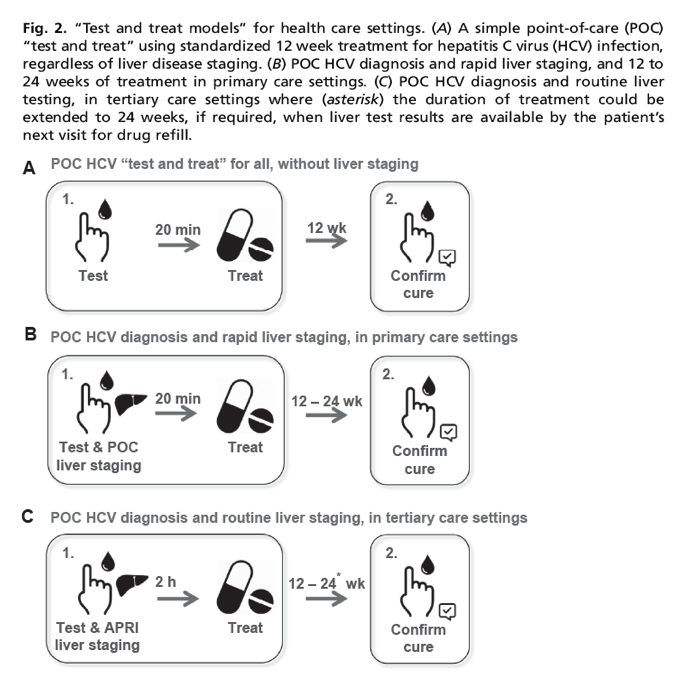
From a public health perspective, HCV diagnosis and care could be simplified to just two visits: (1) diagnosis of active HCV infection and standardized treatment regardless of disease stage, and (2) confirmation of cure posttreatment completion (Fig. 2A). Considering the high rate of cure, experts in the field are currently debating if confirmation of cure may also be considered unnecessary in the near future. Although liver disease stage restrictions for treatment access are being removed globally,22, 23, 24 liver assessment is important to inform treatment duration for some regimens and for monitoring patients with cirrhosis.25, 26, 27 A lack of access to liver disease assessment, however, should not be considered a barrier and treatment should be provided to all. The integration of noninvasive liver assessment into a 30-minute visit in the primary care setting would require the development of an as yet unavailable, rapid, point-of-care (POC) test to measure aspartate aminotransferase and platelet count to calculate the aspartate aminotransferase to platelet ratio index28, 29, 30, 31 or a transient elastography machine, such as the Fibroscan,32 that is markedly more affordable than current options (Fig. 2B). The integration of liver assessment into a single 2-hour visit in a tertiary health care setting and centralized laboratory, however, is entirely feasible using current laboratory-based technologies (Fig. 2C).
Increasing access to existing hepatitis C virus diagnostics is a public health priority
The world now eagerly awaits the "holy grail" for HCV POC diagnosis-an accurate, simple, rapid, and affordable diagnosis of active HCV infection in a single visit. In the meantime, national programs should develop and prioritize new approaches to scale-up access to existing diagnostic assays, particularly in low- and middle-income countries (LMICs), which account for approximately 80% of the global burden.7 Many opportunities exist that may provide solutions to optimize diagnostic and treatment networks and increase the market attractiveness of existing HCV diagnostic technologies to make testing more affordable. Efforts to accurately define the local epidemic, provide access to globally representative validation data for quality assured diagnostics, streamline national regulatory and registration processes, demonstrate real-world demand to support business cases, and facilitate the procurement of affordable products are all required.
Supporting Efforts to Define the Local Hepatitis C Virus Epidemic to Design Comprehensive Testing Strategies
The design and development of a cost-effective, evidence-based national HCV testing strategy is extremely complex and many countries will likely require support to navigate this process to meet elimination targets. The first step, predicated on national stakeholders having an understanding of the local key affected populations and drivers of transmission, is of course impossible without access to quality assured, high-performing assays and well-established testing services (Fig. 3A). This foundation is missing in a large number of countries and must be conducted in close consultation with community groups to gain acceptance and participation as well as a comprehensive understanding of the local context and the needs of those affected. Innovative funding mechanisms to support national surveillance studies that generate preliminary in-country estimates, such as the generic protocol developed by WHO33 and develop strong laboratory networks, should be further explored. Additionally, the development of an expert panel to provide guidance to local nongovernmental organizations, ministries, and implementers to appropriately design and interpret small epidemiologic studies could help to build local capacity and expedite the generation of this needed data. Such input could also be linked to an open access database where survey data meeting objective standards could be deposited for further public dissemination, for program planning, research, and market intelligence purposes, among others.
Collectively, access to such epidemiologic information may be further leveraged in combination with the establishment of open access resources that help to identify the ideal diagnostic algorithm for each setting. For example, a practical on-line tool that incorporates local data, including estimated HCV prevalence, key affected populations, geographically relevant assay performance, local product availability, and cost, could build on existing models34, 35 to help national stakeholders identify optimal, cost-effective diagnostic algorithms.36 Such a model could assess whether there are settings in which 1-step diagnosis of active infection, using a particular product, would be a more feasible and impactful approach to reduce transmissions or decrease the burden of disease among a key population.
Streamlining Transparent Processes to Expedite and Encourage Assay Approval and Registration
As a high-risk in vitro diagnostic (IVD), meaning that inaccurate results pose a high risk to public safety, an HCV screening or diagnostic assay undergoes rigorous assessment during review by stringent regulatory authorities (SRA), including members and observers of the International Medical Device Regulators Forum (IMRDF) ICH, or regulatory bodies associated with an ICH member of the International Council for Harmonization (ICH),37 present in Australia, Canada, Europe, Japan, Iceland, Leichtenstien, Norway, and the United States. Comprehensive assessment includes a review of a significant body of evidence of diagnostic performance in clinical samples from the population in which the IVD is intended to be used, independent laboratory evaluation of the product, as well as a site inspection to assess compliance with good manufacturing practice, in some jurisdictions. Although data supporting US Food and Drug Administration registration38 and World Health Organization (WHO) prequalification39 is available through their websites, access to the European Databank on Medical Devices is currently restricted to competent national authorities, creating an unintended barrier to rapid identification of CE-IVD marked assays. It is, therefore, important that validation data from these SRAs be made widely available. It should be noted, however, that the studies supporting the SRAs are generally restricted to laboratory settings and may have limited applicability to other populations, particularly with respect to genotypes 4, 5, and 640 and coinfection with human immunodeficiency virus (HIV), or real-world field use.
Therefore, to facilitate the uptake of quality-assured and accurate testing products in all countries, collaborative efforts are needed to not only undertake regionally representative clinical validation studies, and share validation data through novel mechanisms and systematic reviews,41, 42, 43 but also to establish cohesive international standards44 (Fig. 3B). Although significant inroads have been made through the WHO Prequalification Programme,45 which assesses the quality of IVDs specifically for use in more resource-limited settings, and the likely inclusion of HCV assays on the Essential Diagnostics List46 is laudable, additional mechanisms to expedite and harmonize national assay approval and registration are required. Initiatives such as the POC Early Infant Diagnosis Consortium, in which multicountry data are pooled to accelerate evaluations, may have wider applicability to fill this need.47, 48 The WHO technical Guidance Series,49 intended for manufacturers, provide a framework for national laboratories to design and conduct local validation studies and are valuable resources. However, simple guidance documents on how to generate reliable population-specific in-country HCV diagnostic validation data, similar to the generic protocols produced by the International Diagnostic Centre (London School of Hygiene and Tropical Medicine50) for CD4, HIV viral load, and so on, would also be valuable. Additionally, the development of an open access database of existing real-world performance data collected from peer-reviewed publications, as well as unpublished and government-led studies could increase access to existing validation data. Although this approach would require significant engagement with each country and a large investment of time to ensure data quality, it could be an important step toward improving transparency and increasing access to known high-performing tests. Greater visibility into clinical accuracy might help to consolidate the market around a handful of high-performing HCV screening and diagnostic tests, more akin to the scenario for HIV, rather than the current situation for HCV in which dozens of different tests can be found within an individual country and even greater variability may be seen across a region. Furthermore, the generation of and access to more diverse local performance data may also help to inform the selection of products that are not yet SRA approved, but perform well. When combined, these collaborative, open access processes may provide new opportunities to increase education and awareness of the significant risks associated with purchasing cheaper products of unassured quality and performance from manufacturers other than those indicated on a list of approved assays.
Last, for those companies interested in marketing their product in geographies with an SRA, the approval pathway may be relatively clear, albeit associated with significant costs. However, if a country with an SRA does not mandate that a test, or a sample type for that test, be approved before clinical implementation, this may further reduce the incentive for companies to pursue SRA review and inadvertently lead to the restricted use of key diagnostics. For instance, dried blood spots (DBS) could be an important method of collecting, storing, and transporting samples to centralized facilities. The expedited approval of DBS as an alternative sample type to diagnose and manage HCV infection using existing approved assays that already have DBS-adapted protocols, such as the Abbott RealTime HCV Viral Load assay (Abbott Diagnostics, Abbott Park, IL), Aptima HCV Quant Dx assay (Hologic Inc, Danbury, CT), and COBAS AmpliPrep/COBAS TaqManHCV Test (Roche Molecular Diagnostics, Basel, Switzerland), would be likely to make significant global impact on rates of HCV diagnosis in both higher income countries and LMICs.43, 51 For companies whose target HCV testing market is restricted to LMICs, the need for SRA approval is often less clear and country-specific validation, registration, and approval processes may be vague.52 Unfortunately, national registration in many LMIC may depend on existing and often unreliable package insert data as the sole source of test performance information. Alternatively, countries may require duplicative local assay validation, adding further costs and delays in implementation. Although products that have not pursued SRA approval or WHO prequalification may be considerably less expensive than products that have, their performance often cannot be guaranteed. Because inaccurate diagnostic results can significantly compromise both public health outcomes and individual patient management, the temptation to procure unapproved or unqualified tests by LMIC must be avoided. LMICs could consider requiring SRA approval or meeting diagnostic accuracy specifications through independent externally generated data as part of the national registration or tendering process to ensure implementation of high-quality products.
Demonstrating Real-World Demand to Support Business Cases to Pursue Approval and Registration of Existing Assays
A greater understanding of the demand for HCV diagnostics among LMICs and higher income countries is urgently required to develop strong business cases that justify the investment in the approval, registration, and marketing of existing assays (Fig. 3C). Further clarity around the real-world market could be developed through strong partnerships between community, academics, clinicians, national stakeholders, nongovernmental organizations, and industry. As countries develop national strategic plans and individual stakeholders, such as ministries of health, nongovernmental organizations, and community groups, embark on testing and treatment programs, a forum to share indicative volumes with industry may be helpful. The cost of testing continues to be a key barrier to designing optimal testing programs and opportunities for more transparent and deconstructed pricing structures from manufacturers and service providers are clearly needed.53
A range of local and collaborative regional testing efforts that may lead to higher volume and more predictable demand estimates for specific HCV diagnostic assays should be explored to achieve more competitive pricing. Pricing currently varies greatly between countries, so increased transparency could also enable individual countries to have stronger negotiation power. One approach is to use a coordinated regional forecast that allows suppliers to provide more indicative pricing linked to volumes, while still allowing procurement to be managed through each individual country. Alternatively, more regionally collaborative approaches could be considered to leverage increased volumes for improved bargaining power to achieve lower pricing: (1) pooled procurement among multiple countries is an option, but would need to balance country sovereignty during the process, or (2) external pooled purchasing through a third party is a possibility for negotiation of a more comprehensive volume guarantee based on regional estimates from multiple stakeholders, such as the not-for-profit Global Procurement Fund.54 Last, LMICs should also consider negotiating with diagnostic companies that already offer testing across multiple diseases to provide options for bundle pricing across the test menu to reach threshold volumes for discount pricing, as well as to negotiate alternative procurement models for equipment acquisition, for example, reagent rental.
Each of these mechanisms can be further enhanced by strong patient advocacy to convince companies of the value of investment and to urge governments, funders, and stakeholders to ensure public HCV services are routinely available, accessible, and affordable. For patients, peers and communities to be empowered to have sustainable impact, support materials and education are needed.55
Complementary, centralized laboratories and point-of-care testing services are needed to increase access to care for all
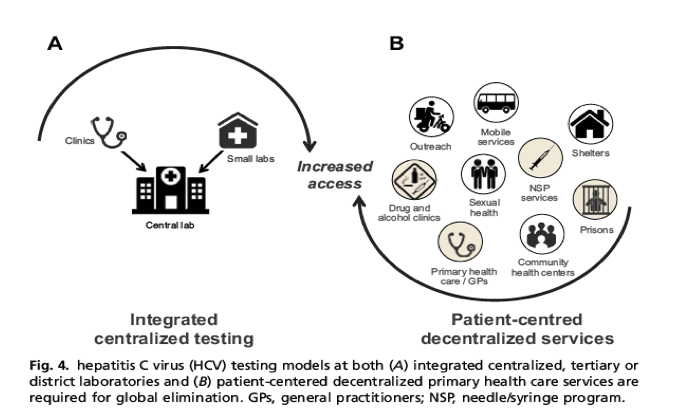
In addition to addressing barriers of limited education, awareness, and health equity, as well as the high stigma and discrimination in ensuring access to HCV care, well-designed testing networks of existing HCV diagnostic tools are needed.56, 57, 58 The elimination of HCV will likely require both centralized laboratories and decentralized POC testing services at community clinics. No single product or testing mechanism is likely to reach all populations affected (Fig. 4A). In addition to patient management, centralized testing is critical for regional surveillance programs to monitor progress toward elimination goals.7 Likewise, as decentralized testing is expanded, countries should also prospectively ensure that data are linked centrally for both surveillance and quality control purposes. Centralized testing also clearly provides advantages in economies of scale, oversight for quality assurance, and data management. Systematic assessment of existing national laboratory testing services (eg, WHO Global Laboratory Initiative and African Society for Laboratory Medicine59), provides countries the opportunity to design improved diagnostic networks and data connectivity. The existing investment in centralized facilities can and should be leveraged to provide efficient and affordable multidisease services.60, 61, 62 New mechanisms to invest in integrated health care systems that address the practical constraints of vertical, disease-specific funding, which can inadvertently limit access to existing infrastructure and equipment, requires strong collaborative commitment from national stakeholders and global funders.60
Decentralized Testing Strategies Are Required to Improve Equitable Access to Hepatitis C Virus Diagnostics for Many Communities
Although providing distinct advantages, centralized testing depends heavily on strong sample transport and result delivery networks, and can delay the time to result. This increase in time consequently increases the number of patient visits and likewise the risk of patient loss along the care cascade. Patient-centered, decentralized testing strategies that empower the patient to control their health care are likely to further reduce the health disparity among communities disproportionately affected by HCV and increase access to HCV diagnosis and care. Decentralized testing services must be adapted to suit a broad range of key affected communities living in urban, regional, rural, and remote settings (Fig. 4B). Task-shifting in these services that allows health care and peer support workers to expand the availability of testing will be critical to scale-up HCV diagnosis.63, 64, 65, 66 Patient-centered approaches embedded within existing services, such as primary care clinics,67, 68, 69 services for the homeless community,70 men who have sex with men,71 sex workers,72 correctional facilities,73, 74 specialist drug services,75, 76, 77 and needle and syringe programs,78, 79 among others, have clearly demonstrated improved access to key populations who may otherwise not be reachable by centralized services.
Several sampling techniques, including oral fluid or fingerstick capillary blood, can facilitate the decentralization of diagnostics.80, 81, 82 Fingerstick capillary blood provides a particularly important sample collection method among many people who inject drugs, for whom poor past health care experiences when accessing veins for standard phlebotomy can remain a huge barrier.83 Capillary blood from a fingerstick can be also collected onto filter paper as a DBS, as a strategy to increase access to centralized HCV testing among people who inject drugs or those in rural or remote populations.51, 84, 85, 86 Studies are underway in the Netherlands87 and Australia (NCT02102451) to assess the potential for self-collected DBS samples as a tool to increase screening, confirm cure, and help monitor reinfection88 among those at risk. Sample collection by finger prick in these settings also provides a unique opportunity for POC HCV RNA testing to provide an immediate result and treatment to the patient.89, 89, 90 Existing near-POC/POC platforms, for example, the CE-marked GeneXpert or Genedrive HCV RNA assays, currently require plasma or serum, but their rational placement and availability could assist to make faster diagnosis more widely available to ensure better testing coverage and promote improved linkage to follow-up services.
Global efforts to find the holy grail: the search for a point-of-care hepatitis C virus diagnostic for active hepatitis C virus infection must continue
Detection of the Hepatitis C Virus Is the Only Test Required to Confirm Active Infection
HCV diagnosis of active infection through direct detection of the HCV virus is the only test required for patient treatment and care. Despite this, HCV is currently diagnosed in 2 steps: first through the detection of HCV antibodies (anti-HCV) using either centralized laboratory tests or rapid diagnostic tests to determine exposure to the virus25, 26, 27; and, second, among those who are anti-HCV positive, active HCV infection is confirmed by nucleic acid testing (NAT) or HCV core antigen (HCVcAg) detection.91 The need for anti-HCV screening followed by HCV RNA and/or HCVcAg testing is entirely driven by the relative costs of each test. As a result, this 2-step diagnostic algorithm is likely to continue until the costs of HCV RNA or HCVcAg tests are significantly reduced. Although anti-HCV rapid diagnostic tests are easy to use and currently provide an affordable screening strategy in many settings,78, 82, 92 multiple studies have demonstrated that up to 25% to 50% of people who are anti-HCV positive fail to return for follow-up NAT to diagnose active infection.93, 94, 95 Concerns also remain around the sensitivity of the anti-HCV assays, particularly in the presence of HIV or hepatitis B coinfection, in other immunocompromised patients, or owing to other poorly understood regional differences in assay performance, although current data remain limited.96, 97 Although reflex testing, in which anti-HCV antibody-positive samples are automatically referred to undergo HCV RNA testing without the need for a separate sample collection visit, has been rolled out in the United States and the UK, limited data are available to demonstrate an impact on improving the retention of people in care and increasing cure in the era of DAA therapy.98, 99 Furthermore, this strategy still relies on centralized testing and, therefore, diagnosis cannot be accomplished in a single visit. Modeling data clearly demonstrate that a shift to a 1-step diagnosis of active HCV infection, without HCV antibody screening, is central to achieving HCV elimination goals.100
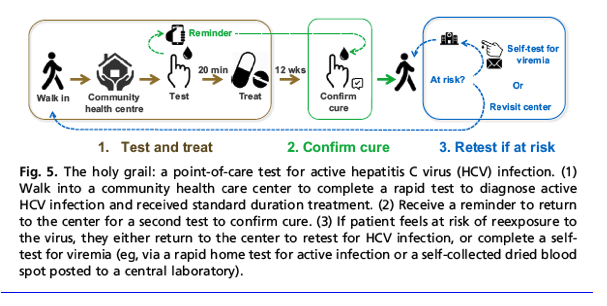
The Holy Grail Point-of-Care Diagnostic Assay for Active Hepatitis C Virus Infection That Links All People with Hepatitis C Infection to Immediate Care Is Essential to Achieve
Global Elimination
To eliminate HCV by 2030 as a public health concern, HCV care will need to be simplified to "test and treat" for all. In the perfect world, simple "test and treat" facilities would be embedded within existing decentralized community services to enable the diagnosis of millions of people with HCV infection and link them immediately into care. The holy grail diagnostic assay would enable a person to walk into a local community health care center without stigma, provide a simple sample, receive their diagnosis of active HCV infection, and start treatment immediately-all in a single visit in under 30 minutes (Fig. 5). Patients would then return to the center to confirm cure. Those with advanced liver disease and/or those who are at risk of reexposure would revisit the center for continued monitoring and care. Alternatively, more regular self-testing could occur at home to screen for reinfection. In this ideal setting, the diagnostic assay would test for active infection and require only a finger prick capillary blood sample or self-collected oral fluid to be loaded directly onto the platform by any health care worker or the patient themselves. The results would be available in 20 minutes and be simple to interpret accurately. Although the platform would ideally be instrument free, a small handheld device or a small, portable instrument may also be fit for this purpose. Any power required by the device would be provided by a long-life, solar-powered, rechargeable battery. If performed using a smart phone or small instrument, the platform would have the capacity to capture and, where connectivity was available, automatically transmit data to facilitate data collection and surveillance reporting. For platforms with this connectivity, the assay could automatically link the patient to their preferred notification tool as a reminder to complete a posttreatment follow-up visit to confirm cure and assist continuity of care. This follow-up visit could occur either at the clinic or through a self-testing option as preferred. Although likely unachievable, the perfect assay that would allow global scale access to POC diagnosis of active infection would cost less than $US5 per test (includes reagent cost only, at scale, ex-works), although consensus on this remains controversial.101
Several Existing Assays and Point-of-Care Platforms Could Be Readily Adapted to Diagnose Active Hepatitis C Virus Infection
Unlike the saturated market for anti-HCV rapid diagnostic tests,102, 103 the GeneXpert HCV Viral Load test (Cepheid, Sunnyvale, CA) and the Genedrive HCV IVD kit (Genedrive Diagnostics, Manchester, UK) are the only SRA-approved near-POC, plasma-based assays that detect active HCV infection. Cepheid is currently the only supplier in LMIC of a multianalyte, integrated platform that uses single-use cartridges to extract, amplify, and detect the presence of HCV by fluorescent reverse transcriptase polymerase chain reaction (PCR).89, 104, 105 However, there are several similar nucleic acid platforms that also use PCR amplification and fluorescent detection that are in development, for example, the TrueNAT Chip-based Real-Time micro PCR test (Diagnostics Molbio Pvt Ltf, Goa, India), or could be adapted to diagnose HCV from existing systems, such as the Enigma Minilab (Enigma Diagnostics Ltd., San Diego, CA)106 and DxNA GeneSTAT System (DxNA LLC, St George, UT). Likewise, several simplified platforms that use alternative amplification technologies, such as reverse transcriptase loop-mediated isothermal amplification, also have commercially-available assays for other viral infections including the Alere i and Alere q for HIV 1/2 Early Infant Diagnosis (Abbott Diagnostics).107, 108, 109 The isothermal reaction within reverse transcriptase loop-mediated isothermal amplification reduces time to result, minimizes platform complexity, and is amenable to a visual result.110The addition of HCV assays to already approved molecular POC platforms would greatly improve competition in the field and help to reduce prices, likely in a more expedient fashion than the launch of a new product platform. In addition, the development of a true POC NAT that diagnoses active HCV infection from finger stick whole blood outside of a laboratory setting is still yet to be realized.
In addition to NAT, a considerable amount of evidence has been generated to support the clinical utility of the HCVcAg in plasma as a marker of active infection. HCVcAg is a viral protein released into the circulation during viral assembly and offers a potentially more stable, alternative marker to HCV RNA for diagnosis of active infection. Currently, there is only 1 CE-IVD marked test for the quantification of HCVcAg in plasma samples for HCV diagnosis and treatment monitoring: the Architect HCV Ag performed on the ARCHITECT Immunoassay Analyser (Abbott Diagnostics) is suitable for centralized testing facilities. Unfortunately, access to the ARCHITECT remains limited in many settings. Therefore, because the instrument footprint for PCR often already exists and the bundled pricing of HCV RNA tests continues to be reduced in LMIC (US$14-$25), NAT is likely to continue to be a more affordable option for LMIC, at least in the short term. However, centralized HCVcAg testing may provide a more affordable option for middle-income countries where platforms are already in use and upfront investment is not required.42, 111, 112, 113, 114 More recent efforts, including by the Center for Innovation in Global Health Technologies (Northwestern University), Abbott Diagnostics, and Qoo Labs (San Diego, CA) have focused on adapting the immunoassay detection of the HCVcAg into a rapid diagnostic lateral flow test, although these platforms are often challenging115 and are still in early stages of prototype development.116
New Classes of Technologies May Transform the Hepatitis C Virus Point-of-Care Landscape in the Next Decade
Although very few new classes of POC diagnostics have come to the market in recent years,117 the world can expect the arrival of transformational technologies in the next few years. Fundamental advances in each diagnostic assay component are underway, including new assay chemistries and nanotechnologies to improve sample capture and detection, and novel materials and microfluidics to allow miniaturization.118, 119, 120 Smart phone-assisted diagnosis of infection, through either the adaptation of reading devices or addition of biosensing platforms to a mobile device, are also on the horizon and promise to improve access to testing, including in rural and remote communities.121, 122 Investments to maximize smart phone diagnostic innovations may provide opportunities to improve health care more broadly, including improved surveillance data collection in remote and resource-limited settings, increased telehealth capacity, and the ability to more easily implement interventions to enhance linkage to care.123 The availability of new materials and innovative solutions such as 3-dimensional printing are also likely to further reduce costs and facilitate the scale-up of many of these technologies.124, 125 Although many of these new classes of diagnostic tools have been developed for other viral infections, such as HIV or Zika, these advances can undoubtedly be quickly translated to HCV diagnosis and management if there is commitment.
Mechanisms to Further Stimulate Investment in the Development of Novel Diagnostics Suitable for Resource Limited Settings Are Essential
Although promising new, transformational diagnostic technologies are on the horizon, novel mechanisms to decrease costs and risks to further stimulate investment in research and development are needed.126, 127 Although the diagnostic development pathway is relatively low cost and low risk when compared with drug development, the successful penetration of a new diagnostic assay into the market can take at least 7 years and needs to overcome 2 "valleys of death" before launch.128, 129 There can also be an overwhelming lack of interest to develop diagnostic assays suitable for LMIC where the market has not been strongly developed, despite potentially high volumes. Considering that 80% of those with chronic HCV infection live in LMIC, it is imperative to find new funding models to accelerate the development of commercially viable, fit-for-purpose assays for LMIC if elimination goals of HCV are to be achieved.
Several approaches may be considered to decrease the cost and risk associated with investing in a diagnostic product (Fig. 6). Diagnostic companies could reassess the unmet need and business advantages of focusing on the development of assays for multiple diseases on integrated, single platforms to improve efficiencies and increase profits. Another strategy may be to implement differential pricing that allows the sale of premium priced assays within high-income countries to support the sale of reduced priced assays in LMICs. Although there are not many examples of this previously being successful, the fact that POC/1-step diagnosis of HCV is applicable in both higher income countries and LMIC settings may present a unique situation where the same product can be viable in both markets. Another possibility would be for drug and diagnostic companies to find synergy: although, at least in theory, it would seem to be in the interests of drug companies to invest in companion diagnostics that identify those in need of their treatment, unfortunately, the profit margins remain concentrated in high-income countries so there is currently little incentive for originator drug companies to invest in affordable diagnostics suitable for LMICs. More recently, generic drug companies are exploring partnerships with diagnostic suppliers or developing companion diagnostics to offer bundled pricing and bridge the diagnosis-to-treatment gap in LMICs.55 However, considering the potential for this approach to introduce product monopolies where a diagnosis may only be offered in concert with a single drug supplier, this strategy would need to be assessed, regulated, and monitored carefully. Diagnostic companies can also be constrained by current treatment guidelines,25, 27, 130 which define strict lower limits of quantitation to guide patient management. These guidelines are based on currently available analytical thresholds for HCV RNA assays rather than the real-world clinical sensitivity required for effective patient management.131 Because analytical HCV RNA thresholds are likely considerably lower than clinically relevant thresholds, a global systematic review of the distribution of HCV RNA among real-world cohorts has been commissioned by FIND and WHO to help define the lower qualitative HCV RNA thresholds required for effective patient management and update current target product profiles for new qualitative nucleic acid diagnostics.101 Although ensuring testing guidelines keep pace in a rapidly changing therapeutic environment can be challenging, this strategy to review analytical and clinical requirements may help to further encourage industry to invest in the development of simple and affordable diagnostic solutions.
Last, innovative funding mechanisms to drive program scale up are needed. Proposals such as the Global Alliance for Medical Diagnostics Initiative could prove valuable, but will require significant funding commitments and careful management around governance, independence and conflict of interests.132 Recent initiatives such as the Stop TB Partnership "Venture Lab" (vLab) provide examples of how private-public partnerships may be able to accelerate and scale up diagnostics.133 Innovative approaches to provide needed initial funding to galvanize large-scale national public health HCV programs as well advocacy to global donors, such as PEPFAR and the Global Fund, to support diagnosis and treatment of HCV will be key. Commitments from these funders would send very powerful signals to suppliers that a viable market is achievable.
Summary: remaining challenges for global access to hepatitis C virus diagnostics
It is time the world took HCV diagnostics seriously. Wide-spread diagnosis of HCV is essential to achieve global elimination and increasing access is now a public health priority. The global community must come together to collectively support efforts to develop testing strategies that are patient-centric, expedite assay registration, reduce costs, and generate the demand required for businesses to invest in wide-scale roll out of existing products. No one diagnostic solution will fit all purposes, but the global community must invest in partnerships that facilitate the development and introduction of the holy grail POC HCV diagnostic test to ensure finding the missing millions in need of curative treatment.
| |
| |
| |
|
|
|