| |
|
Transplanting Hepatitis C Virus-Infected Versus Uninfected Kidneys Into Hepatitis C Virus-Infected Recipients: A Cost-Effectiveness Analysis
|
| |
| |
Download the PDF here
Download the PDF here
10 July 2018
"Conclusion:
Transplanting HCV-infected kidneys into HCV-infected patients increased quality-adjusted life expectancy and reduced costs compared with transplanting HCV-uninfected kidneys into HCV-infected patients."
"In our analysis, the superiority of transplanting HCV-infected kidneys depends on the high efficacy of new interferon-free regimens. This net benefit persists despite the slightly higher risk for death in the 30 days after transplant of infected kidneys. In sensitivity analyses, we found that 30-day mortality for these patients would have to increase considerably from base-case values before transplant of HCV-uninfected kidneys would be preferred."
------------------------
"The recent availability of direct-acting antivirals that can be used in ESRD has created new opportunities and questions regarding the optimal timing of treatment of chronic HCV infection in patients awaiting kidney transplant. Despite our newfound ability to treat such patients before transplant, our analysis shows that the benefit of earlier transplant afforded by using HCV-infected kidneys outweighs the risk for progressive liver disease due to untreated HCV infection while awaiting transplant. Additional wait times for HCV-uninfected kidneys would have to decrease below 161 days before treating with uninfected kidneys would be preferred. We believe that our analysis supports transplanting HCV-infected kidneys into patients with ESRD who already are infected with HCV, and we hope that these results will be used to guide decision making by individual patients and treatment centers. Nevertheless, given the wide range of wait times at different centers, we recognize that some patients will prefer transplant of HCV-uninfected kidneys preceded by treatment of HCV infection.
We analyzed an alternative scenario in which all patients received glecaprevir-pibrentasvir. Recipients of HCV-uninfected kidneys, regardless of genotype, were treated for 8 or 12 weeks depending on the absence or presence, respectively, of compensated cirrhosis. As in the base case, all patients receiving treatment after transplant of an HCV-infected kidney received 12 weeks of treatment regardless of genotype. Transplanting an infected kidney resulted in a similar gain (0.50 QALY) at a smaller lifetime cost savings ($19 762) compared with transplanting an uninfected kidney. We also explored the effect of additional wait time to receive an HCV-uninfected kidney in this scenario. Although transplanting an uninfected kidney was more effective and less expensive if there was no additional wait time, transplanting an infected kidney dominated (that is, was more effective and less costly) beyond a wait time of 0.45 year (164 days).
In the past, treatment of HCV infection in transplant recipients has been limited by interferon-mediated graft rejection and poor efficacy. Recent studies of direct-acting antivirals in kidney transplant recipients have shown high rates of sustained virologic response with minimal adverse events or graft rejection (45). However, experience is still limited, and some physicians have raised concerns about the safety and efficacy of HCV therapy for transplant recipients (46). A recent meta-analysis of 6 studies involving 360 renal transplant recipients found that 98.3% of patients achieved sustained virologic response within 12 weeks. [pdf attached above] Roughly 1% of patients had significant adverse events (47). In our analysis, the superiority of transplanting HCV-infected kidneys depends on the high efficacy of new interferon-free regimens. This net benefit persists despite the slightly higher risk for death in the 30 days after transplant of infected kidneys. In sensitivity analyses, we found that 30-day mortality for these patients would have to increase considerably from base-case values before transplant of HCV-uninfected kidneys would be preferred.
- Pungpapong et al[41] found that 72% of 25 patients who received DAAs developed anemia that required intervention or dose reduction. Overall, unlike IFN-α, DAAs are effective cures for HCV infection and do not impair liver and renal function, and avoid acute rejection, allograft loss, and serious AEs. -
In another sensitivity analysis, we explored the effect of decreasing the cost of all direct-acting antiviral agents by up to 50%. Transplant of an HCV-infected kidney continued to dominate the analysis; however, at a cost reduction of 50%, savings decreased to $36 402. In an analysis examining the effect of patient age, deferred antiviral therapy continued to dominate. At younger ages, cost savings were lower but the gain in effectiveness was larger (for example, at age 30 years, cost savings was $37 735 and effectiveness 0.58). At older ages, cost savings increased but the gain in effectiveness decreased (for example, at age 70 years, cost savings was $44 701 and effectiveness 0.43). In sensitivity analyses exploring the effects of sex and race, results changed little. We also considered an alternative scenario in which the patient already had compensated cirrhosis. Transplanting HCV-infected kidneys continued to dominate the analysis, but the gain in effectiveness was smaller (0.25 QALY). In this scenario, transplanting an infected kidney increased the lifetime probability of dying of end-stage liver disease to 19% (vs. 14% in patients receiving an HCV-uninfected kidney) but decreased that of dying of chronic kidney disease to 26% (vs. 32%)."
---------------------------------------------------------
Transplanting Hepatitis C Virus-Infected Versus Uninfected Kidneys Into Hepatitis C Virus-Infected Recipients: A Cost-Effectiveness Analysis
Annas Int Med - 10 July 2018 - Mark H. Eckman, MD, MS; E. Steve Woodle, MD; Charuhas V. Thakar, MD; Flavio Paterno, MD, MPH; Kenneth E. Sherman, MD, PhD
Abstract
Background:
Direct-acting antiviral agents are now available to treat chronic hepatitis C virus (HCV) infection in patients with end-stage renal disease (ESRD).
Objective:
To examine whether it is more cost-effective to transplant HCV-infected or HCV-uninfected kidneys into HCV-infected patients.
Design:
Markov state-transition decision model.
Data Sources:
MEDLINE searches and bibliographies from relevant English-language articles.
Target Population:
HCV-infected patients with ESRD receiving hemodialysis in the United States.
Time Horizon:
Lifetime.
Perspective:
Health care system.
Intervention:
Transplant of an HCV-infected kidney followed by HCV treatment versus transplant of an HCV-uninfected kidney preceded by HCV treatment.
Outcome Measures:
Effectiveness, measured in quality-adjusted life-years (QALYs), and costs, measured in 2017 U.S. dollars.
Results of Base-Case Analysis:
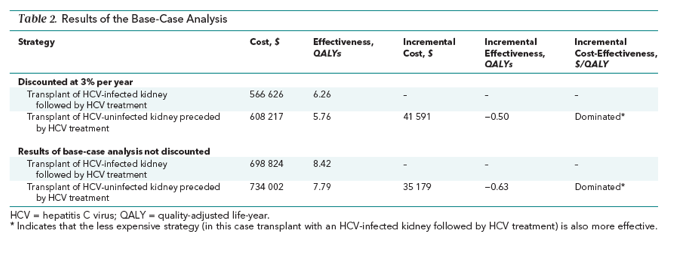
Transplant of an HCV-infected kidney followed by HCV treatment was more effective and less costly than transplant of an HCV-uninfected kidney preceded by HCV treatment, largely because of longer wait times for uninfected kidneys. A typical 57.8-year-old patient receiving hemodialysis would gain an average of 0.50 QALY at a lifetime cost savings of $41 591.
Results of Sensitivity Analysis:
Transplant of an HCV-infected kidney followed by HCV treatment continued to be preferred in sensitivity analyses of many model parameters. Transplant of an HCV-uninfected kidney preceded by HCV treatment was not preferred unless the additional wait time for an uninfected kidney was less than 161 days.
Limitation:
The study did not consider the benefit of decreased HCV transmission from treating HCV-infected patients.
Conclusion:
Transplanting HCV-infected kidneys into HCV-infected patients increased quality-adjusted life expectancy and reduced costs compared with transplanting HCV-uninfected kidneys into HCV-infected patients.
Primary Funding Source:
Merck Sharp & Dohme and the National Center for Advancing Translational Sciences.
An estimated 110 000 U.S. patients start dialysis each year. In 2016, approximately 500 000 patients received dialysis for end-stage renal disease (ESRD), of whom 19 060 (3.8%) received kidney transplants. Because of limited organ availability, hemodialysis is the final treatment for most patients with ESRD. The scarcity of kidneys for transplant and high mortality rate while awaiting the procedure have led some physicians and patients to consider transplanting organs that otherwise might not be considered. For example, between 10% and 15% of U.S. patients receiving dialysis are seropositive for hepatitis C virus (HCV) (1). Some of these patients are willing to accept HCV-infected kidneys from deceased donors (2), in part because wait times for such kidneys are shorter than those for HCV-uninfected kidneys (average, 469 vs. 856 days [3]). Because the yearly mortality rate for patients receiving hemodialysis is 4% to 16% (4, 5), reducing the time to kidney transplant can have a dramatic effect on overall survival and quality of life. Moreover, the U.S. Food and Drug Administration approved elbasvir-grazoprevir in January 2016 and glecaprevir-pibrentasvir in August 2017 for treating chronic HCV infection in patients with ESRD (6, 7). The availability of these drugs has created opportunities for transplanting HCV-infected kidneys into patients who already have HCV infection, but it has also introduced an interesting dilemma. Should patients with chronic HCV infection receive an HCV-uninfected kidney, which means that they would be treated for HCV infection before the transplant and would have to wait longer for the procedure? Alternatively, should they receive an HCV-infected kidney, with treatment for HCV infection after the transplant and a shorter wait time? We developed a decision analytic model to estimate the comparative effectiveness and cost-effectiveness of these 2 options.
Results
Transplanting an HCV-infected kidney followed by HCV treatment decreased quality-adjusted life-years (QALYs) by increasing the lifetime probability of dying of end-stage liver disease and related causes to 5%, compared with 3.4% for transplanting an HCV-uninfected kidney preceded by HCV treatment. However, transplanting an uninfected kidney preceded by treatment decreased QALYs by increasing the duration of dialysis while waiting for a kidney and thus increasing the lifetime probability of dying of chronic kidney disease to 34.5%, compared with 29% for patients receiving HCV-infected kidneys followed by HCV treatment (Supplement Figures 3 and 4). The net benefit of transplanting an infected kidney followed by HCV treatment was a survival gain of 0.50 QALY at a lifetime cost savings of $41 591 compared with transplant of an HCV-uninfected kidney preceded by HCV treatment (Table 2).
Deterministic Sensitivity Analysis
Using an HCV-infected kidney and deferring treatment was more effective and less costly than using an HCV-uninfected kidney preceded by HCV treatment in sensitivity analyses within clinically plausible ranges or 95% CIs for most model parameters.
Some model parameters were more sensitive to variations in other parameter values. Additional wait time to receive an HCV-uninfected kidney may vary substantially from center to center and patient to patient. Figure 1 (top) explores variations in this wait time between 0 and 1.6 years compared with the average wait time to receive an HCV-infected kidney. In the base case, average wait time for an infected kidney is 0.63 year (231 days) and the additional time required to receive an uninfected kidney is 1.48 years (540 days). Transplant of an HCV-uninfected kidney preceded by HCV treatment is not preferred unless the additional wait time for an uninfected kidney decreases to less than 0.44 years (161 days). Figure 1 also shows that the incremental cost-effectiveness ratio of transplanting an uninfected kidney preceded by HCV treatment compared with transplanting an infected kidney followed by treatment is roughly $50 000 per QALY when the additional wait time is 0 and exceeds $100 000 per QALY beyond an additional wait time of 0.2 year (73 days).
We also explored the additional risk for death associated with transplanting HCV-infected kidneys. As shown in Figure 1 (bottom), transplant of infected kidneys followed by antiviral therapy is best unless 30-day mortality after transplant exceeds 10% (base case, 1.5%) for patients receiving infected kidneys versus 0.85% for those receiving HCV-uninfected kidneys.
Figure 2 depicts a 2-way sensitivity analysis of annual excess mortality after transplant of a kidney from a deceased donor (horizontal axis) and annual excess mortality among patients receiving hemodialysis (vertical axis). The diagonal threshold line shows that transplant of an uninfected kidney preceded by HCV treatment could be the preferred strategy if annual excess mortality for patients receiving hemodialysis were substantially lower and if excess mortality after transplant were higher. The base-case values fall well within the region in which transplanting HCV-infected kidneys is preferred.
Figure 3 shows a 2-way sensitivity analysis of patients' quality of life while receiving hemodialysis (horizontal axis) and after kidney transplant (vertical axis). Transplant of an uninfected kidney preceded by HCV treatment would be preferred only if, compared with base-case values, the patient's quality of life while receiving dialysis were substantially higher and quality of life after transplant were substantially lower.
We analyzed an alternative scenario in which all patients received glecaprevir-pibrentasvir. Recipients of HCV-uninfected kidneys, regardless of genotype, were treated for 8 or 12 weeks depending on the absence or presence, respectively, of compensated cirrhosis. As in the base case, all patients receiving treatment after transplant of an HCV-infected kidney received 12 weeks of treatment regardless of genotype. Transplanting an infected kidney resulted in a similar gain (0.50 QALY) at a smaller lifetime cost savings ($19 762) compared with transplanting an uninfected kidney. We also explored the effect of additional wait time to receive an HCV-uninfected kidney in this scenario. Although transplanting an uninfected kidney was more effective and less expensive if there was no additional wait time, transplanting an infected kidney dominated (that is, was more effective and less costly) beyond a wait time of 0.45 year (164 days).
In another sensitivity analysis, we explored the effect of decreasing the cost of all direct-acting antiviral agents by up to 50%. Transplant of an HCV-infected kidney continued to dominate the analysis; however, at a cost reduction of 50%, savings decreased to $36 402. In an analysis examining the effect of patient age, deferred antiviral therapy continued to dominate. At younger ages, cost savings were lower but the gain in effectiveness was larger (for example, at age 30 years, cost savings was $37 735 and effectiveness 0.58). At older ages, cost savings increased but the gain in effectiveness decreased (for example, at age 70 years, cost savings was $44 701 and effectiveness 0.43). In sensitivity analyses exploring the effects of sex and race, results changed little. We also considered an alternative scenario in which the patient already had compensated cirrhosis. Transplanting HCV-infected kidneys continued to dominate the analysis, but the gain in effectiveness was smaller (0.25 QALY). In this scenario, transplanting an infected kidney increased the lifetime probability of dying of end-stage liver disease to 19% (vs. 14% in patients receiving an HCV-uninfected kidney) but decreased that of dying of chronic kidney disease to 26% (vs. 32%).
Probabilistic Sensitivity Analysis
Over 10 000 iterations, transplanting HCV-infected kidneys followed by HCV treatment was preferred over transplanting HCV-uninfected kidneys preceded by HCV treatment 100% of the time, yielding an average gain of 0.52 QALY (SD, 0.16) (Supplement Figure 5) at an average cost savings of $38 691 (SD, $9141). Transplanting infected kidneys was less costly and more effective—that is, it dominated transplanting uninfected kidneys 99.99% of the time and was cost-saving or had an incremental cost-effectiveness ratio less than $50 000 per QALY 100% of the time.
Discussion
The recent availability of direct-acting antivirals that can be used in ESRD has created new opportunities and questions regarding the optimal timing of treatment of chronic HCV infection in patients awaiting kidney transplant. Despite our newfound ability to treat such patients before transplant, our analysis shows that the benefit of earlier transplant afforded by using HCV-infected kidneys outweighs the risk for progressive liver disease due to untreated HCV infection while awaiting transplant. Additional wait times for HCV-uninfected kidneys would have to decrease below 161 days before treating with uninfected kidneys would be preferred. We believe that our analysis supports transplanting HCV-infected kidneys into patients with ESRD who already are infected with HCV, and we hope that these results will be used to guide decision making by individual patients and treatment centers. Nevertheless, given the wide range of wait times at different centers, we recognize that some patients will prefer transplant of HCV-uninfected kidneys preceded by treatment of HCV infection.
In the past, treatment of HCV infection in transplant recipients has been limited by interferon-mediated graft rejection and poor efficacy. Recent studies of direct-acting antivirals in kidney transplant recipients have shown high rates of sustained virologic response with minimal adverse events or graft rejection (45). However, experience is still limited, and some physicians have raised concerns about the safety and efficacy of HCV therapy for transplant recipients (46). A recent meta-analysis of 6 studies involving 360 renal transplant recipients found that 98.3% of patients achieved sustained virologic response within 12 weeks. Roughly 1% of patients had significant adverse events (47). In our analysis, the superiority of transplanting HCV-infected kidneys depends on the high efficacy of new interferon-free regimens. This net benefit persists despite the slightly higher risk for death in the 30 days after transplant of infected kidneys. In sensitivity analyses, we found that 30-day mortality for these patients would have to increase considerably from base-case values before transplant of HCV-uninfected kidneys would be preferred.
Our analysis has several limitations. Our base case considered an “average” patient with chronic HCV infection awaiting a kidney transplant. We did not consider more specific patient characteristics or comorbid conditions. For instance, patients with diabetes mellitus have higher mortality rates while receiving hemodialysis and after kidney transplant (5). Thus, a more individualized approach may be warranted for some patients. We did not address the issue of co-infection with hepatitis B virus or HIV. Treatment of kidney transplant recipients who are co-infected with HIV and HCV requires continued awareness and attention to the complex drug interactions that can occur among direct-acting antivirals, antiretroviral medications, and immunosuppressive medications. Reactivation of hepatitis B viral infection has been reported in patients starting direct-acting antiviral therapy for HCV who are not receiving medications for hepatitis B virus infection. We also assumed that the small fraction of patients in whom initial HCV treatment failed would not receive salvage therapy. Studies in posttransplant patients are limited for this rapidly evolving area. We did sensitivity analyses in which we provided high-efficacy therapy for treatment-experienced patients, and results were not substantially different from those of our base case. Finally, we did not consider the benefits of decreased prevalence of HCV infection in dialysis units and decreased HCV transmission that would result from treating HCV-infected patients who are receiving dialysis.
In summary, the availability of interferon-free direct-acting antivirals to treat chronic HCV infection in kidney transplant recipients creates a new opportunity to provide access to HCV-infected kidneys for HCV-infected patients with ESRD. Doing so can greatly reduce the wait time for a donated kidney and improve survival by decreasing time spent receiving hemodialysis. In an era of increasing success for kidney transplants and demand that far outstrips supply, deferring antiviral therapy until after transplant of HCV-infected kidneys, when available, should be both cost-saving and effective.
| |
| |
| |
|
|
|