| |
Variable HIV Life Expectancy
|
| |
| |
Download the PDF
from Jules: What you see in this analysis & discussion on life expectancy is that studies are estimates and suggest variability in life expectancy with many variables I think relevant. You cannot extrapolate from studies of estimates what the future will bear particularly that these studies cannot & do not include comorbidities; these studies do not estimate outcomes if patients have comorbidities, and its impossible to predict even if they did because the outcomes of comorbidities will vary from person to person. My experience is that there are groups of HIV+ who have relatively intact health and appear to live well into their early 70s. Even still, these individuals have some health issues like earlier heart disease, hearing loss. Others are suffering with numerous conorbidities including 3-6 at a time that include any from a basket of diseases like: bone disease & fractures; cardiovascular disease & heart attack; any number of different cancers - although cancer rates are low they are greater among HIV compared to HIV-negatives; cognitive impairment, mental health, depression, anxiety & neurologic disease including neuropathy are present for a large number of older aging HIV+. Frailty occurs at earlier ages in HIV+ and occurs more often in HIV+. You can see below link to study from Amsterdam AGEhIV group frailty was close to 20% in HIV+ over 65 compared to around 5% in HIV-negatives, but HIV+ individuals are also much more likely to have 1 or 2 of the frailty components like weakness, slowness. Quality of life is not discussed here but is key to HIV+ who have more depression, worse frailty rates, more isolation, worse mental health, more comorbidities, worse polyphrmacy, more problems with poverty, income & housing; less health literacy is common among HIV+ and more cognitive impairment and these make it more difficult to navigate the healthcare system for a population who will need it and use more because they have so many more comorbidoties. Poverty, health literacy, and cognitive impairment & depression are factors in life expectancy.
"HIV infection is independently associated with frailty in middle-aged HIV type 1-infected individuals compared with similar but uninfected controls"
http://www.natap.org/2016/HIV/011516_01.htm
These are excerpts from: Epidemiology of ageing with HIV: what can we learn from cohorts? Sabin, Caroline A.; Reiss, Peter, AIDS June 2017
It is now accepted that the majority of PLWH who start treatment with combination antiretroviral therapy (cART) will have a rapid virological response and a good immunological recovery on treatment. This has resulted in dramatic improvements in life expectancy in PLWH in many settings. Using data from the UK Collaborative HIV Cohort (UK CHIC) Study, May and colleagues [2] reported that life expectancy of PLWH at age 20 had increased from 30.0 to 45.8 years from 1996-1999 to 2006-2008. However, despite this increase, overall life expectancy was only 39.5 for men and 50.2 years for women compared with 57.8 and 61.6 years for men and women in the general population (1996-2006). Thus, at the time of the study, life expectancy remained about 13 years less than that of the general UK population. Using data from the Danish HIV Cohort Study, Legarth et al. [3] reported that median survival time from age 50 years among PLWH had increased from 11.8 years during 1996-1999 to 22.8 years from 2006-2014. However, when compared with an individually matched cohort from the background population, mortality rates remained substantially higher (from 3.8 times higher in those aged 50-55 years to 1.6 times higher in those aged 75-80 years). Among a subset of the cohort of 517 well treated PLWH without AIDS or existing comorbidities, median survival time from age 50 years was 25.6 years compared with 34.2 years in the matched general population sample. In a second study from the Danish HIV Cohort, Rasmussen et al. [4] reported that the proportion of PLWH aged more than 50 years increased from 13% in 1995 to 43% in 2014. With the exception of nonvirus/smoking-associated cancers, PLWH had a higher excess and relative risk of all age-related diseases than did the controls. Similar trends have been reported from the United States and Canada [5], where life expectancy at age 20 increased from 36.1 to 51.4 years from 2000-2002 to 2006-2007. Worldwide, although estimated life expectancy has improved in recent years, PLWH continue to have an estimated life expectancy at age 20 years that is 10.9-39.7% lower than that of the general population, depending on the geographic setting [6].
In an updated analysis from the UK CHIC study [7], life expectancy among PLWH receiving cART was found to depend heavily on the attained CD4 cell count and viral load; after 5 years on ART, the expected age at death of a 35-year-old man varied from 54 years (if the man's CD4+ cell count was <200 cells/μl and he had no viral suppression) to 80 years (if his CD4+ cell count was ≥350 cells/μl and he had viral suppression). Importantly, life expectancy in men and women with good CD4+ and viral load responses appeared to be at least as good as, if not better than, that in the general population. The possibility of improved life expectancy in some subgroups had previously been reported by the Collaboration of Observational HIV Epidemiological Research in Europe (COHERE) collaboration [8], a large European collaboration of cohort studies. In this study, mortality rates among those on cART with a CD4+ cell count of at least 500 cells/μl were similar to those of the general population in noninjection drug using men [standardized mortality ratio when compared with the general population 0.9 (95% confidence interval 0.7-1.3)] and, after 3 years of cART, in women [1.1 (0.7-1.7)].
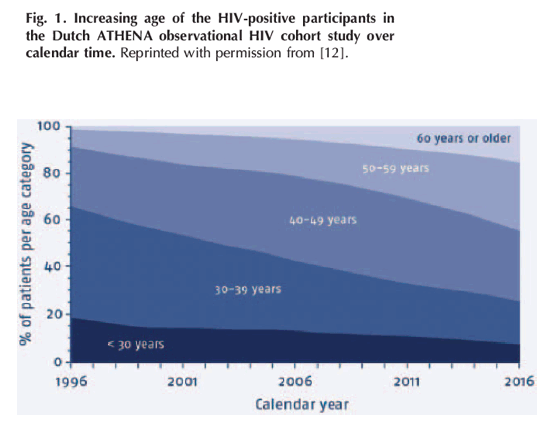
In addition to the increased life expectancy of PLWH, there have also been continued new HIV diagnoses (and new HIV infections) in those aged more than 50 years. Data from Public Health England show that the proportion of people newly diagnosed with HIV who were over the age of 50 had increased from 9.2% in 2005 to 18.6% in 2014 [9]. Among the 733 adults known to be newly infected in 2014 (as determined by the Recent Infection Testing Algorithm), 9.7% were aged 50 or older [10]. The impact of these changes in life expectancy and patterns of new diagnoses has been dramatic. Within Europe, the proportion of new HIV diagnoses among individuals aged 50 or over has increased from 7.4% in 2005 to 12.0% in 2014 [11]. Anecdotally, many HIV clinics have reported that the average age of those attending their clinics has increased, with the proportion of attendees over the age of 50 increasing dramatically (e.g. Fig. 1, which shows the changing age distribution of participants in the Dutch national ATHENA study over time [12]). Globally, the estimated number of PLWH who are thought to be aged over 50 years has increased from 650 000 in 1990 to 5 800 800 in 2015 (http://aidsinfo.unaids.org). But these increases in age are also expected to continue into the future: using information from the ATHENA study, Smit et al. [13] constructed an individual-based model of the ageing HIV-positive population, which predicted that the median age of patients receiving treatment for HIV will increase in the Netherlands from 43.9 years in 2010 to 56.6 years in 2030, with the proportion of patients aged more than 50 years predicted to increase from 28 to 73% over the same period.
What are the major consequences of an ageing clinic population?
As the cohort of PLWH has aged, and the incidence of AIDS-related conditions has reduced with effective cART, so the spectrum and burden of comorbidities in the cohort has increased. This has been most noticeable when considering patterns of mortality. When deaths do occur, these now tend to be in older people and the causes of death are different to those previously reported. In the Swiss HIV Cohort study [14], AIDS-related mortality peaked in 1992 and decreased to a low level in 2006. Compared with deceased persons in 2005, those who died only 4 years later in 2009 tended to be older (49 vs. 45 years at death), with a higher CD4+ cell count (321 vs. 257 cells/μl), less likely to be ART naive (5 vs. 13%), and less likely to have died of AIDS-related causes (9 vs. 23%); 84% of all deaths occurring between 2005 and 2009 were from non-AIDS-related causes. In France, Morlat et al. [15] reported a drop in the proportion of deaths among PLWH that were because of AIDS-related causes, with an increase in the proportion of deaths from non-AIDS-defining malignancies and cardiovascular disease (CVD) compared with earlier years. Similar findings were reported from the large, multinational Data Collection on Adverse Effects of anti-HIV Drugs (D:A:D) study [16], with a reduction over time in the proportion of deaths from AIDS-related causes being mirrored by an increase in the proportion of deaths from cancers. The lack of increase in the proportion of deaths from CVD in this study was likely because of improvements in interventions to prevent or treat CVD, particularly in those who had a primary myocardial infarction (MI) in whom survival had improved [17].
There has also been an increase in the number of PLWH who are also living with comorbidities. Among participants in the Dutch AGEhIV Cohort study, HIV-positive participants had a mean of 1.3 age-associated noncommunicable comorbidities (AANCC) in comparison to only 1 in demographically matched HIV-negative controls [18]. Hypertension, MI, peripheral arterial disease, and impaired renal function were all significantly more prevalent among the HIV-positive participants in the study. Notably, the comorbidity profile of HIV-positive participants in this cohort, more closely matched that of an HIV-negative control that was 5-10 years older. Similar associations have been reported from other European settings [19]. With the continued increase in the age distribution of PLWH, the prevalence of comorbidities is expected to increase, with Smit et al. [13] predicting that by 2030, 84% of PLWH will have at least one AANCC (an increase from 28% in 2010), with 28% of PLWH expected to have three or more such AANCC. As a consequence, it is estimated that 54% of PLWH will be prescribed comedications in 2030 compared with 13% in 2010, with 20% taking three or more comedications. The implications of this for clinic costs have been studied in one cohort in Canada [20]; in this cohort, the proportion of older patients increased from 9.6 to 25.4% from 1999 to 2010. Over this time, older patients incurred higher costs for all aspects of HIV care such that by 2010, the proportion of clinic costs incurred by older patients had increased from 25 to 31%. More expensive ART as a consequence of more complex regimens, more comorbid interactions and greater adherence accounted for most of the cost difference.
Of the AANCC that have been described, CVD and cancers are arguably the most commonly studied. CVD is of particular concern in PLWH given the documented associations with some antiretroviral drugs as well as the high, and increasing, prevalence of known risk factors for CVD in the HIV-positive population [21]. The prevalence of dyslipidaemia and hypertension are increasing in most cohort populations, largely as a result of the ageing population although ART-associated effects on lipids and blood pressure (BP) and immunosuppression/exposure to HIV viraemia may also contribute [22-24]. The jury is still out on whether these key risk factors for CVD have the same impact on subsequent MI rates as in the general population. In the Veterans Aging Cohort Study Virtual Cohort [25], hazard ratios associated with various degrees of hypertension (compared with those with normal BP levels) appeared to be greater in those with HIV than in HIV-uninfected controls, suggesting that the combination of HIV and hypertension may confer a greater risk of MI than that conferred by hypertension alone. Among participants in the Swiss HIV Cohort Study [26], insufficient control of hypertension was associated with increased risk of cardiovascular events, indicating the need for improved management of hypertension in PLWH.
The literature around the cancer field remains somewhat inconsistent. In primarily black African clinic cohorts in Maryland, there was a rapid increase in the incidence of new cancers, which was driven largely by an increase in the incidence of non-AIDS-defining cancers, with the incidence of AIDS-defining cancers remaining relatively stable [27]. In contrast, an early meta-analysis conducted by Shiels et al. [28] showed that, compared with the general population, there had been an increased rate of many non-AIDS-defining cancers, notably anal cancer, Hodgkin's lymphoma, liver, stomach, and lung cancers, in both the cART and non-cART eras. Interestingly, there was a reduced risk of both prostate and breast cancers in those with HIV, possibly reflecting more close monitoring in this group. In a more recent analysis from the French Hospital's Database on HIV in which the authors restricted the population to those who had been on cART with a good CD4+ cell count response, there did not appear to be any increased risk of lung cancer compared with expected rates from the general population, but rates of both Hodgkin's lymphoma and liver cancer remained elevated [29]. An updated analysis of the Veterans Aging Cohort Study reported that while the overall all-cancer crude incidence rate had increased between 1997-2000 and 2009-2012, once rates were standardized for age changes, there were significant declines in PLWH in the rates of all cancer, AIDS-defining cancers, non-AIDS-defining cancers, and nonvirus-related non-AIDS-defining cancers [30].
|
|
| |
| |
|
|
|