| |
HIV Accelerates Brain Damage ;
"Neurological Complications of HIV Infection" Review
|
| |
| |
Download the PDF here
From Jules: This has been controversial over the past 1 year with several conflicting studies presented at CROI. Karl Goodkin published MACS analyze finding HIV accelerates brain aging that these authors say is a good reliable study. This is a very important statement. i have said for years HIV accelerates brain damage but it is not always easy to show in a study. I remain convinced that at least for some & probably many HIV accelerates aging brain damage AND THIS gets little to no attention, this finding will translate into a serious development over the next 5 years as many HIV+ age further to beyond 65.
1. "Does higher penetrance of antiretroviral drugs into the central nervous system reduce HAND through enhanced control of CNS infection? Or does higher cART penetrance in the CNS promote HAND through drug toxicity to neurons? These are open questions to which considerable attention has been paid in the last 5 years."
2. most recent large-scale study to tackle this question, by Goodkin and colleagues....used the prospective Multicenter AIDS Cohort Study....offers convincing evidence that aging accelerates HIV's deleterious effects on the specific neurocognitive domains of episodic memory and motor function,,,,The study was limited by discrepancies in race and educational level between the seropositive and uninfected groups, but nevertheless offers convincing evidence that aging accelerates HIV's deleterious effects on the specific neurocognitive domains of episodic memory and motor function. Crucially, the authors show the importance of controlling for the duration of HIV infection, which should be included in future studies of the impact of age on HIV-associated morbidities.
Goodkin MACS Study - HIV prematurely ages the brain - new study & Commentary - (07/19/17)
Progressive Brain Atrophy Despite Persistent Viral Suppression in HIV Over Age 60 - (07/19/17)
3. Studies of two novel drugs, cenicriviroc and natalizumab, offer hope that blockade of cellular trafficking may lead to improvements in HAND. Cenicriviroc is a dual CCR2 and CCR5 antagonist that is proposed to reduce monocyte activation and trafficking into the CNS [64].....Using an SIV model of infection, Campbell and colleagues showed that natalizumab prevented viral seeding of the CNS when administered to rhesus macaques during acute SIV infection and was associated with fewer monocytes in the CNS, lower levels of soluble CD163, and decreased neuronal injury on magnetic resonance spectroscopy [66]. This offers very exciting proof of concept that blocking cellular trafficking during acute infection may prevent neurological consequences of HIV disease.
4. "HIV enters the central nervous system during acute infection through trafficking of infected immune cells across the blood-brain barrier, initiating a process of CNS infection and immune activation that can be detected through CSF markers of inflammation and brain imaging [14, 15]. Genetic analysis of virus isolated in CSF in patients early in HIV infection shows that, initially, CNS HIV shares sequence similarity to blood virus but, over time, evolves independently into a compartmentalized CNS virus......Two recent studies in humanized myeloid-only mice demonstrated replication competent virus in tissue macrophages, including in the brain [18 , 19]. This is an important proof of concept for the ability of HIV to persist in microglial cells, even in the absence of T cells. This is an important proof of concept for the ability of HIV to persist in microglial cells, even in the absence of T cells.....Ultimately, early introduction of HIV into the CNS may establish a reservoir of infection that continues to drive neuroinflammation even after cART is initiated......even among individuals with durable plasma viral suppression, HIV RNA remains detectable in the CSF in about 10–40% of cases (depending on the sensitivity of the detection assay), a phenomenon termed "CNS escape" [22 , 23].....When CNS viral sequences are examined in patients with CNS escape on suppressive therapy, sequence divergence can be detected between CNS and plasma virus, suggesting a role for compartmentalized virus in driving ongoing neuroinflammation [22 ]......Numerous studies point to persistent immune activation in the CNS during cART. CSF neopterin, a marker of macrophage activation, remains elevated in patients on suppressive cART.
.......Although CNS escape, or the identification of viral replication in CSF during plasma viral suppression, is relatively rare, autopsy studies reveal that HIV DNA is present in the brain even during systemically suppressive cART. ....... showed that HIV DNA was detected in more than half of the brain tissue samples they analyzed, alongside a wide range of brain pathologies (only a few of which were considered classic for HIV-associated neurologic disease) [25 ].
----------------
Neurological Complications of HIV Infection
Curr Infect Dis Rep Dec 2017 - Shelli Farhadian1 & Payal Patel 2 & Serena Spudich3
Abstract
Purpose of Review
HIV-associated neurocognitive disorders (HAND) are common in patients with HIV disease, even during suppressive combination antiretroviral therapy (cART). This review article addresses the pathogenesis of HAND, focusing on important findings from the last 5 years.
Recent Findings
While HIV-associated dementia is now rare in settings with cART availability, mild forms of HAND are increasing in prevalence. Biomarkers of cellular injury, such as neurofilament light chain and neopterin, can detect early stages of neuroinflammation associated with HIV infection and are increased even in asymptomatic individuals with chronic HIV infection. Several recent studies form a growing body of evidence that HIV can infect and replicate in monocytes and that blocking monocyte activity can potentially improve neurological outcomes in HIV. Early cART may also prevent HAND.
Summary
Understanding the multifactorial causes of CNS infection and inflammation is critical to devising treatment and preventive strategies for HAND.
INTRODUCTION
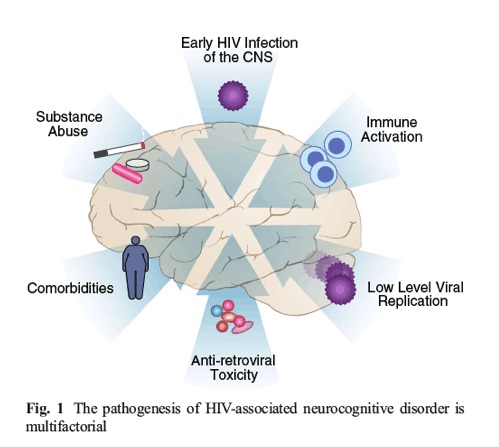
As individuals with HIV continue to benefit from major advances in HIV treatment, patients now present with the sequelae of aging with a chronic viral infection. Among the most prevalent of these chronic HIV-associated, non-AIDS conditions are neurocognitive impairment. This review will describe the phenomenon of HIV-associated neurocognitive disorders (HAND), an umbrella term that refers to deficiencies in memory, concentration, attention, and motor skills in individuals with HIV. We describe clinical features of HAND and review recent studies that aim to understand the mechanisms of neurocognitive deficits in chronic HIV infection in the era of combination antiretroviral therapy (cART) (Fig. 1).
Clinical features of HAND
Prior to the widespread use of cART, advanced HIV disease was associated with high rates of neurological disease, including neuropathy and encephalopathy [1]. Rates of HIV-associated dementia have declined precipitously since the routine adoption of suppressive cART, but the prevalence of all neurocognitive disorders in HIV remains unchanged: an estimated 50% of patients with HIV on cART have some form of HAND [2, 3]. This is due to an increase in the number of patients living with milder forms of HAND: mild neurocognitive disorder (MND) and asymptomatic neurocognitive impairment (ANI).
MND and ANI are research designations and are currently assessed through neuropsychological testing and self-reported impairment in daily functioning (Table 1). While there is some controversy regarding the clinical significance of these milder forms of neurocognitive disease, a recent study shows an increased risk of progression to symptomatic neurocognitive impairment in patients with ANI, as well as ongoing neuroinflammation in these participants [4 ]. This suggests that ANI reflects true neuropathology and offers the potential for early intervention to prevent worsening cognitive impairment.
Laboratory Markers of HAND
Although the diagnosis of HAND currently requires formal neuropsychological testing, there are several cerebrospinal fluid (CSF) biomarkers that have been associated with neurocognitive impairment in HIV and which might be candidates for future diagnostic tests for HAND. Neopterin is a marker of macrophage activation and is elevated in CSF of untreated patients with HIV-associated dementia [5]. Neurofilament light chain (NFL) is a marker of axonal injury that is predictive of severity and survival in several neurocognitive diseases [6]. In HIV, high levels of CSF NFL are found not only in patients with HIV-associated dementia, but also, intriguingly, in individuals who are neurologically asymptomatic but with low CD4 counts [7], suggesting chronic neuronal injury even in this asymptomatic population. cART reduces but does not normalize levels of CSF NFL [8]. Both neopterin and NFL are elevated in participants with ANI and MND [9 ], and a recent study suggests that blood NFL correlates well with CSF NFL, making this a particularly attractive candidate for early diagnosis of HAND [10]. Other potential biomarkers for HAND include the CSF/plasma albumin ratio (a marker of blood-brain barrier breakdown) [11], as well as blood biomarkers associated with Alzheimer's disease, such as p-tau and Aβ42, though the evidence for these remains controversial [12, 13]. Further studies are needed to validate the significance of Alzheimer's disease biomarkers in individuals with HIV.
Mechanisms of CNS Injury
Early Events in Infection
HIV enters the central nervous system during acute infection through trafficking of infected immune cells across the blood-brain barrier, initiating a process of CNS infection and immune activation that can be detected through CSF markers of inflammation and brain imaging [14, 15]. Genetic analysis of virus isolated in CSF in patients early in HIV infection shows that, initially, CNS HIV shares sequence similarity to blood virus but, over time, evolves independently into a compartmentalized CNS virus. This CNS virus is more typically macrophage tropic, allowing it to persist in CNS perivascular macrophages and monocytes despite predictably low levels of CD4+ T cells in this environment [16]. An ongoing question is whether HIV is trafficked to the CNS via infected monocytes (in addition to infected T cells), or whether CNS virus evolves to more efficiently infect monocyte-derived cells once it is already in the CNS. It is also unknown whether HIV productively infects parenchymal microglial cells, the embryonically derived tissue macrophages of the CNS [17]. Two recent studies in humanized myeloid-only mice demonstrated replication competent virus in tissue macrophages, including in the brain [18 , 19]. This is an important proof of concept for the ability of HIV to persist in microglial cells, even in the absence of T cells.
Once infected, CNS perivascular macrophages and perhaps microglial cells release inflammatory cytokines and neurotoxins that perpetuate neuronal injury. In contrast, neurons, oligodendrocytes, and astrocytes do not appear to be productively infected by HIV. Ultimately, early introduction of HIV into the CNS may establish a reservoir of infection that continues to drive neuroinflammation even after cART is initiated.
"CNS Escape"/Chronic Viral Replication in the CNS
During uncontrolled HIV infection, there is chronic, low-level invasion of virus into the CNS, most of which is trafficked to the CNS through migrating infected T cells and possibly monocytes, and some of which may be produced locally in CNS tissue [20]. In untreated patients, the concentration of HIV detected in CSF is about one tenth that of plasma during chronic infection [21]. Controlling HIV infection in peripheral blood, then, is the most effective way of preventing ongoing migration of infected lymphocytes to CNS. However, even among individuals with durable plasma viral suppression, HIV RNA remains detectable in the CSF in about 10–40% of cases (depending on the sensitivity of the detection assay), a phenomenon termed "CNS escape" [22 , 23].
Closer examination of patients with CNS escape has yielded important information about the potential pathogenesis of HAND. Although most patients with CNS escape are neurologically asymptomatic, they do produce higher levels of CSF neopterin, a biomarker of macrophage activation, suggesting a chronic state of neuroinflammation in these individuals that may underlie later neurologic disease [24]. When CNS viral sequences are examined in patients with CNS escape on suppressive therapy, sequence divergence can be detected between CNS and plasma virus, suggesting a role for compartmentalized virus in driving ongoing neuroinflammation [22 ].
Although CNS escape, or the identification of viral replication in CSF during plasma viral suppression, is relatively rare, autopsy studies reveal that HIV DNA is present in the brain even during systemically suppressive cART. By using PCR to examine various tissues in post-mortem participants with HIV and documented plasma viral suppression, Lamers and colleagues showed that HIV DNA was detected in more than half of the brain tissue samples they analyzed, alongside a wide range of brain pathologies (only a few of which were considered classic for HIV-associated neurologic disease) [25 ]. Though many of these individuals had malignancies or other conditions that might affect HIV persistence, the detection of HIV DNA nevertheless offers a further clue to the persistence of HIV in the brain of patients with seemingly controlled infection.
Chronic Immune Activation in the CNS
Numerous studies point to persistent immune activation in the CNS during cART. CSF neopterin, a marker of macrophage activation, remains elevated in patients on suppressive cART. Yilmaz and colleagues monitored CSF neopterin in participants on cART and found that 41% of patients had persistently abnormal levels of CSF neopterin even after a decade of cART [26]. Neuroimaging studies also suggest persistent immune activation in the CNS during cART [27].
The cellular nature of neuroinflammation in suppressed HIV remains an active area of research. Most descriptions of the CNS inflammatory milieu in HIV are based on flow-cytometry studies of CSF. These studies have revealed that T lymphocytes in the CSF (as in blood) in HIV are predominantly CD8+, resulting in a lowered CD4+:CD8+ ratio compared to uninfected individuals in the CSF compartment [28, 29]. A lower CSF CD4+:CD8+ ratio is in turn associated with worsened neurocognitive impairment in participants with HIV [30]. Chronic HIV is associated with a depletion of naïve T cells and an increase in the proportion of memory T cells [29, 30] and of activated T cells, in blood and in CSF [29, 30, 31 ]. Suppressive cART reduces but does not normalize levels of T cell activation, and participants with more severe neurocognitive impairment have the highest rates of activated T cells in CSF [30].
Recent studies have more specifically characterized the pool of activated CD8+ T cells in the CSF. In a Thai-based cohort of participants with acute HIV infection, Kessing and colleagues demonstrated that a subset of CD8+ T cells in the CSF specifically targets HIV antigens and does so at higher rates when compared to the pool of CD8+ T cells in the periphery [32]. In a separate study, HIV controllers, i.e., individuals who are able to maintain low to undetectable plasma viremia without cART, were also found to have HIV-specific CD8+ T cells in the CSF [31 ]. More research is needed to understand whether virus-specific CD8+ T cells in CSF play a role in persistent neuroinflammation during chronic HIV infection in individual on cART.
Toxicity/Penetrance of cART
Does higher penetrance of antiretroviral drugs into the central nervous system reduce HAND through enhanced control of CNS infection? Or does higher cART penetrance in the CNS promote HAND through drug toxicity to neurons? These are open questions to which considerable attention has been paid in the last 5 years. To address this issue, the CNS penetration-effectiveness (CPE) score was developed as a means to quantify drug penetrance to the CNS and to research associations between ART penetrance in the CNS and resultant neuronal injury. Drug regimens with good CNS penetration, i.e., those with high CPE scores, have been found to be more effective in controlling CSF viral load compared to regimens with low CPE scores [33]. Current first-line ART regimens suggested by the U.S. Department of Health and Human Services all achieve a CPE rank of 7–8, indicating excellent CNS penetration.
Certain ART regimens are associated with worse cognitive outcomes, possibly due to toxicity from CNS exposure rather than incomplete penetrance of the drug. This has been particularly well described in the case of efavirenz, which is associated with high rates of neuropsychiatric impairment and subsequent drug discontinuation [34, 35, 36]. Further studies are needed to clarify the effects of specific cART regimens on CNS HIV infection. This is particularly urgent in light of recent efforts to simplify drug regimens from three to two active agents, where relatively reduced exposure of antiretroviral agents in the brain as compared to blood might lead to adverse CNS consequences [37, 38].
Neurocognition and "Premature Aging" in HIV
Studies of older individuals with HIV suggest that individuals with HIV disease are at higher risk for non-AIDS conditions such as renal disease, certain malignancies, cardiovascular disease, and neurocognitive impairment than the general populations [39, 40, 41]. This has led to the hypothesis that HIV causes accelerated or "premature" aging. However, epidemiological studies offer conflicting evidence on whether individuals with HIV disease acquire these non-AIDS conditions at a younger age than their uninfected counterparts, leading to significant controversy around the subject of accelerated aging in HIV [39, 42, 43]. Studies on HIV and aging that focus on neurocognitive impairment as a primary outcome have also yielded mixed results [44, 45, 46]. These studies have generally been limited by an inability to control for the severity and duration of HIV infection, by difficulties controlling for confounding risk factors, and by cross-sectional rather than longitudinal study design. The most recent large-scale study to tackle this question, by Goodkin and colleagues, has overcome many of these limitations to address whether age and HIV act synergistically to affect neurocognitive decline [47 ]. This study used the prospective Multicenter AIDS Cohort Study, which includes longitudinal neurocognitive data from > 47,000 visits in > 5000 individuals, and excluded individuals with a history of CNS opportunistic infections, tumor, severe substance use disorder, and major co-morbidities. The study was limited by discrepancies in race and educational level between the seropositive and uninfected groups, but nevertheless offers convincing evidence that aging accelerates HIV's deleterious effects on the specific neurocognitive domains of episodic memory and motor function. Crucially, the authors show the importance of controlling for the duration of HIV infection, which should be included in future studies of the impact of age on HIV-associated morbidities.
Long-Term Neurocognitive Effects of pediatric HIV Infection
Perinatally acquired HIV affects over two million children worldwide with 160,000 new infections occurring every year. A majority of children living with HIV reside in developing countries and have limited access to antiretroviral therapies [48]. In developed countries, the rate of new perinatally acquired HIV infections has fallen dramatically due to better access to HIV testing and therapy for pregnant women [49]. Additionally, survival of adolescents and young adults living with HIV has improved because of cART. These factors have led to an increased global prevalence of HIV-related neurologic complications in young individuals.
Several key features of perinatally acquired infections are important to the pathogenesis of HIV-related neurologic complications in children. In infancy, the developing blood-brain barrier is more permeable and susceptible to injury from infectious processes [50]. White matter signal abnormalities have been found in young children who have initiated antiretroviral therapy before 3 months of age indicating early cerebral injury related to HIV despite appropriate therapy [51]. Synaptic formation and pruning occurring throughout childhood may also be affected by HIV exposure, as evidenced by subtle white matter tract changes in HIV-positive children classified as slow progressors [52]. Neurologic manifestations of HIV in pediatric patients include cerebrovascular disease, epilepsy, and most commonly, cognitive issues [53]. Cognitive impairment in children with HIV is broad, usually encompassing multiple domains, and more severe in children with a history of AIDS-defining illnesses [54, 55].
Prospects for HAND Prevention & Treatment
cART improves cognitive performance in antiretroviral naïve patients with established HAND [56, 57], but it is still unknown whether cART can preventHAND by reducing CNS viral compartmentalization and immune activation. Recent studies have begun to examine the potential benefits of early initiation of cART, during acute HIV infection, in preventing HAND. Using a Peru-based cohort of individuals with acute and recent (<3 months) HIV infection, Robertson and colleagues randomized study participants to immediate or deferred (24 weeks) cART and monitored neuropsychologic outcomes at baseline and following treatment. They found better neurocognitive performance at 48 weeks in the immediate treatment group as compared to the deferred treatment group [58]. Similarly, Evering et al. found low rates of HAND among a small (n = 26) cohort of men who started cART during primary infection, though this study was limited by the lack of a control group for comparison testing [59]. In our own Thai-based cohort of participants with acute HIV infection, we found that 6 months of cART initiated during acute HIV was associated with preserved good performance in the majority of participants, with improvement on repeated testing largely paralleling the performance in a group of HIV-uninfected Thais. However, a subset with impaired performance on neuropsychological testing and a high CSF viral load at baseline showed no benefit of early treatment after 6 months [60]. These findings suggest a possible role for early cART in preventing neuro-HIV disease in participants who are cognitively normal at baseline.
The choice of a "best" cART regimen to prevent or treat HAND remains unknown. A randomized trial of participants with HAND who were initiating or switching cART asked whether participants who were placed on a drug regimen with a high CPE score (i.e., highly penetrant to the CNS) had improved outcomes when compared to participants placed on a regimen with a low CPE score [61]. The drugs that were most frequently used in the high-CPE group were emtricitabine, zidovudine, and lopinavir. The authors found no difference in cognitive outcomes between the two groups, though both showed improved cognitive performance after 16 weeks of viral suppression on cART. Small studies looking at treatment intensification, through the addition of maraviroc [62] or maraviroc plus raltegravir [63] to a standard cART regimen have found conflicting results as to whether treatment intensification yields a cognitive benefit in patients with virologic suppression and established HAND. Randomized clinical trials are needed to definitively address these issues.
Studies of two novel drugs, cenicriviroc and natalizumab, offer hope that blockade of cellular trafficking may lead to improvements in HAND. Cenicriviroc is a dual CCR2 and CCR5 antagonist that is proposed to reduce monocyte activation and trafficking into the CNS [64]. It is currently in an interventional phase II study (NCT02128828) to evaluate safety and efficacy in treating mild-moderate HAND. Preliminary data from 17 participants in a single-arm pilot study show that cenicriviroc decreases soluble markers of monocyte activation and is associated with improved cognitive performance after 24 weeks [65]. Natalizumab is an antibody against the α4 subunit of α4β1 and α4β7 integrins, which are expressed on lymphocytes and monocytes and are required for migration of these cells from the periphery to the CNS. Using an SIV model of infection, Campbell and colleagues showed that natalizumab prevented viral seeding of the CNS when administered to rhesus macaques during acute SIV infection and was associated with fewer monocytes in the CNS, lower levels of soluble CD163, and decreased neuronal injury on magnetic resonance spectroscopy [66]. This offers very exciting proof of concept that blocking cellular trafficking during acute infection may prevent neurological consequences of HIV disease.
CONCLUSIONS
Despite remarkable advances in the treatment of HIV disease, the prevalence of HIV-associated neurocognitive disorders remains high. This is likely due to multiple factors, including cART use and chronic immune activation even during virologic suppression. Recent studies have shed further light on the potential to treat and prevent HAND using cART and have elucidated immune mechanisms that contribute to HAND and which may form the basis for further targeted therapy.
|
|
| |
| |
|
|
|