| |
Switch from tenofovir disoproxil fumarate combination to dolutegravir
with rilpivirine improves parameters of bone health
|
| |
| |
Download the PDF here
AIDS Feb 20 2018 - McComsey, Grace A.a; Lupo, Sergiob; Parks, Davidc; Poggio, Monica Coronadod; De Wet, Josephe; Kahl, Lesley P.f; Angelis, Kostasg; Wynne, Brianh; Vandermeulen, Katii; Gartland, Martinj; Cupo, Michaelk; Aboud, Michaelfon behalf of the 202094 Sub-Study Investigators
Objective: Bone mineral density (BMD) loss, a risk factor for osteoporosis, has been attributed to HIV infection and antiretroviral therapy (ART), including regimens containing tenofovir disoproxil fumarate.
Design: Study 202094 is an open-label, parallel-group, sub-study of the phase III SWORD-1 and SWORD-2 studies (ClinicalTrials.gov identifier, NCT02478632).
Methods: HIV-1-infected adults with HIV-1 RNA less than 50 copies/ml who received ART containing tenofovir disoproxil fumarate for at least 6 months were randomized to receive dolutegravir with rilpivirine or continue current ART regimen. Total hip and lumbar spine BMD were measured by dual-energy X-ray absorptiometry (DXA) scans. The primary endpoint was percentage change from baseline in total hip BMD.
Results: DXA scans were evaluable for 81 participants at baseline and Week 48. Percentage increase in total hip BMD was significantly greater in participants who switched to dolutegravir with rilpivirine (1.34%) compared with participants who continued current ART (0.05%; treatment difference, +1.29%; 95% CI 0.27-2.31; P = 0.014). Lumbar spine BMD significantly increased in the dolutegravir with rilpivirine group by 1.46% (95% CI 0.65-2.28) compared with 0.15% (95% CI -0.79 to 1.09) in the current ART group (treatment difference, 1.32; 95% CI 0.07-2.57; P = 0.039). Participants in the dolutegravir with rilpivirine group experienced significantly greater reductions in bone formation and resorption biomarkers compared with the current ART group.
Conclusion: Switch to dolutegravir with rilpivirine was associated with significant improvement in BMD and bone turnover markers compared with tenofovir-based three-drug regimens, providing a robust option for preserving bone health while continuing suppressive ART.
Introduction
Bone disease is an important comorbidity in the aging HIV population [1-3]. Numerous studies have shown that patients with HIV experience a decline in bone mineral density (BMD) and higher bone fracture incidence compared with the general population [4]. A meta-analysis of cross-sectional studies reported pooled odds ratios of 6.4 for reduced BMD and 3.7 for osteoporosis in HIV-infected compared with non-HIV-infected patients [5]. In addition, a large study that evaluated data in a United States healthcare system revealed overall fracture prevalence of 2.87% in HIV-infected patients compared with 1.77% in non-HIV-infected patients [5]. Loss of BMD in patients with HIV is associated with many contributing factors including antiretroviral therapy (ART), particularly regimens including tenofovir disoproxil fumarate [5,6]. Mechanisms that may cause ART-associated BMD loss remain uncertain but may include mitochondrial toxicity, urinary phosphate wasting, and renal osteodystrophy [7]. Reports have described improved BMD after switching from regimens containing tenofovir disoproxil fumarate to regimens containing other nucleoside reverse transcriptase inhibitors (NRTIs) [8,9].
Two-drug regimens (2DRs) are being developed to simplify HIV treatment by using combinations of agents that retain virologic efficacy comparable with that of three-drug regimens but limit toxicity risks [10]. A retrospective observational cohort study showed preliminary support for the efficacy and safety of a regimen constituting the integrase strand transfer inhibitor (INSTI) dolutegravir and the non-NRTI (NNRTI) rilpivirine [11]. Both dolutegravir and rilpivirine have demonstrated high potency for inhibition of HIV-1 in phase III studies [11-17]. The virologic potency and pharmacologic attributes of dolutegravir and rilpivirine led to their selection for development as a 2DR to maintain suppression of HIV-1 [18,19].
In the SWORD-1 and SWORD-2 trials, participants with HIV who were virologically suppressed for at least 6 months were randomized to continue with their current ART regimen or switch to the 2DR of dolutegravir with rilpivirine. This report describes a sub-study (202094) of SWORD-1 and SWORD-2 to evaluate changes at Week 48 in BMD and bone turnover biomarkers after switching from a three-drug regimen containing tenofovir disoproxil fumarate to the NRTI-sparing dolutegravir with rilpivirine regimen.
Results
Study population
Of 151 participants screened, 49 participants were excluded on the basis of inclusion/exclusion criteria (n = 43), investigator discretion (n = 1), lost to follow-up (n = 1), withdrew consent (n = 3), or multiple reasons (n = 1); 102 participants were included in the sub-study (Fig. 1). Twenty-one participants did not have evaluable DXA scans at baseline and at Week 48. Twelve of these participants (dolutegravir with rilpivirine, three; current ART, nine) did not have a baseline DXA scan after Day 15, eight participants (dolutegravir with rilpivirine, four; current ART, four) were withdrawn from the parent study before providing a DXA scan at Week 48, and one participant (current ART) incorrectly switched to dolutegravir with rilpivirine on the day of the Week 48 scan; hence, the scan was excluded from the analysis. Therefore, 81 participants (dolutegravir with rilpivirine, n = 46; current ART, n = 35) had evaluable DXA scans at baseline and Week 48. Among the 12 participants with no evaluable DXA scan at baseline, one current ART participant withdrew after completing the Week 48 DXA scan. Nine participants withdrew in total (dolutegravir with rilpivirine, four; current ART, five). Participant demographics at baseline, including age, ethnicity, sex, BMD, and BMI, were balanced between the two groups (Table 1). Approximately half of the participants in both treatment groups were women, the majority of study participants were nonsmokers and did not consume alcohol, and baseline third-agent classes were mostly NNRTI-based without significant differences between treatment arms. Most study participants in both treatment arms had normal total hip and lumbar spine T scores. Less than 30% were classified as osteopenic, and no participants met the osteoporosis criterion by total hip T score. However, slightly greater than 30% were osteopenic in both treatment arms, and approximately 6% met the osteoporosis criterion by lumbar spine T score (Table 1).
Changes in bone mineral density
The percentage increase in total hip BMD measured by areal density from baseline to Week 48 was significantly greater in participants who switched to dolutegravir with rilpivirine (1.34%) compared with current ART (0.05%; difference in adjusted percentage change, +1.29%; 95% CI 0.27-2.31; P = 0.014; Fig. 2). The percentage increase in lumbar spine BMD from baseline to Week 48 (1.46%) was also significantly greater in the dolutegravir with rilpivirine group compared with the current ART group (0.15%; difference in adjusted percentage change, 1.32; 95% CI 0.07-2.57; P = 0.039; Fig. 2). The significant total hip result was also supported by a significant difference between treatment arms in the adjusted change from baseline to Week 48 in the total hip T score (difference in adjusted percentage change: 0.09; 95% CI 0.02-0.16; P = 0.016). A similar observation was made for the mean difference in adjusted change from baseline to Week 48 in the lumbar spine T score (difference in adjusted percentage change: 0.12; 95% CI 0.00-0.23; P = 0.049). A significantly greater increase from baseline to Week 48 was also observed in total hip and lumbar spine Z scores for the dolutegravir with rilpivirine group compared with the current ART group (P = 0.026 and P = 0.013, respectively).
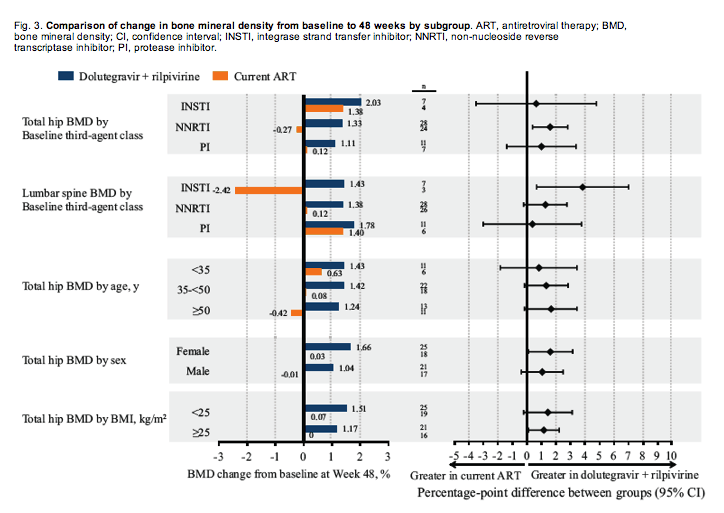
The change from baseline to Week 48 in total hip and lumbar spine BMD, expressed as areal density, was evaluated across demographic subgroups (age and sex) and baseline BMI. These results supported the primary analysis because a greater change from baseline was observed in the dolutegravir with rilpivirine group compared with the current ART group across all demographic subgroups; however, statistical comparisons were limited by the small sample size within each category (Fig. 3). Demographic groups at greater risk for BMD loss (e.g. ≥50 years of age, women, and BMI <25 kg/m2) exhibited greater increases in adjusted percentage change from baseline to Week 48 in total hip BMD in the dolutegravir with rilpivirine group compared with the current ART group (Fig. 3). Additionally, greater increases in point estimates of mean adjusted change from baseline in total hip and lumbar spine BMD were observed in participants in the dolutegravir with rilpivirine group compared with the current ART group regardless of baseline third-agent class (INSTI, NNRTI, or protease inhibitor; Fig. 3). Differences between groups within each baseline third-agent class were not significant, but this may be attributed to the small sample size within each class.
There was little change from baseline to Week 48 for participants in the dolutegravir with rilpivirine or current ART groups in the 10-year probability of hip fracture (-0.08 and 0.03%, respectively) and osteoporotic fracture (-0.12 and -0.04%, respectively) as assessed by FRAX score [20].
A post hoc analysis from baseline to Week 48 showed that participants in the dolutegravir with rilpivirine group had a similar mean change in BMI (0.84 kg/m2) compared with the current ART group (0.62 kg/m2). Vitamin D supplementation was reported for 20 of the 102 participants at baseline and did not change markedly through Week 48, with discontinuation of vitamin D reported for three participants (dolutegravir with rilpivirine, two; current ART, one).
Changes in bone biomarkers
Participants in the dolutegravir with rilpivirine group experienced significantly greater reductions from baseline to Week 48 in bone-specific alkaline phosphatase, osteocalcin, procollagen type 1 N-propeptide, and type 1 collagen cross-linked C-telopeptide compared with the current ART group (P value range from <0.001 to 0.029 across markers; Table 2). These results were consistent with those for concentrations of the same bone turnover biomarkers following analysis of pooled data from the SWORD-1 and SWORD-2 studies (N = 991) [21].
Safety
No adverse events were attributable to the DXA scan procedure. Clinically significant loss of BMD (defined as ≥5%) at Week 48 was reported in one 31-year-old male participant (dolutegravir with rilpivirine group). This participant's BMI was 21.8 kg/m2 at baseline and dropped to 20.5 kg/m2 at Week 48. The participant was a current smoker, had vitamin D levels within the normal range, and did not receive vitamin D or calcium supplementation during the study period. The investigator concluded that pharmacological intervention was not required. One 61-year-old postmenopausal female participant experienced a nontraumatic fracture of the right fibula (current ART group). This was considered an adverse event of moderate intensity but was not related to study treatment. This participant's BMI remained stable in the normal range during the study period; her vitamin D level was 80 nmol/mm3 at baseline, and the level had decreased to 50 nmol/mm3 at Week 48. However, she was osteopenic at baseline, with a T score of -2.01 which further decreased to -2.33 at Week 48.
Discussion
The primary analysis of the 202094 sub-study of the pooled SWORD-1 and SWORD-2 study populations demonstrated that participants who received dolutegravir with rilpivirine had an increase from baseline to Week 48 in total hip (1.34%) and lumbar spine BMD (1.46%), which differed significantly (P = 0.014 and P = 0.039, respectively) from participants who continued to receive ART containing tenofovir disoproxil fumarate. We selected total hip BMD as the primary measurement of interest because hip is composed of more compact cortical bone and less trabecular bone compared with lumbar spine [22]; therefore, change in total hip BMD is the more conservative endpoint because it changes less readily in comparison with lumbar spine BMD. The significant changes in total hip BMD demonstrated a marked positive effect on bone health after switching from tenofovir disoproxil fumarate-containing three-drug regimens to the NRTI-sparing 2DR, dolutegravir with rilpivirine.
Although a limited number of switch studies that replaced tenofovir disoproxil fumarate with other NRTIs like abacavir or tenofovir alafenamide showed a beneficial effect on BMD [8,23], this is the first randomized study to show that a switch from a tenofovir disoproxil fumarate-based regimen to an NRTI-sparing regimen led to a beneficial effect on BMD and bone turnover markers. A small study (n = 37) replaced tenofovir disoproxil fumarate with raltegravir, but this was a nonrandomized study, and many participants remained on an NRTI, often emtricitabine [9].
Data for other secondary endpoints, including evaluation of change in BMD expressed as T and Z scores and evaluation of change in BMD over 48 weeks by baseline third-agent class, supported the primary endpoint analysis. Despite the limited number of participants in some subgroup categories, data fromsubgroup analyses were consistent with and supportive of the primary endpoint analysis. The beneficial effect seems consistent in high-risk populations such as older participants, women, and smokers.
In a post hoc analysis, participants in the dolutegravir with rilpivirine and current ART groups had similar mean BMI values at baseline. We observed small but similar changes from baseline in BMI in both groups at Week 48. As there was no significant treatment effect on BMI at Week 48, the effect of dolutegravir with rilpivirine on total hip BMD at Week 48 is unlikely to be confounded by concurrent changes in BMI.
In addition to the consistent effect on BMD, we observed significant decreases in bone turnover markers after the switch. Bone undergoes constant remodelling, with osteoclasts resorbing older bone and osteoblasts laying down new bone, and the actions of osteoclasts and osteoblasts can be assessed in vivo using bone turnover markers [5]. These processes are normally tightly coupled, but in HIV, especially whenever initiating tenofovir disoproxil fumarate-containing ART, accelerated bone resorption results in net bone loss. In our study, among participants receiving dolutegravir with rilpivirine, the decreases from baseline to Week 48 in levels of bone formation markers (bone-specific alkaline phosphatase, procollagen type 1 N-propeptide, and osteocalcin) and the bone resorption marker type-1 collagen cross-linked C-telopeptide were significantly greater than the decreases in all bone turnover biomarkers in participants who continued current ART. Taken together, these data indicate a lower rate of bone turnover in participants who received dolutegravir with rilpivirine compared with those who continued current ART.
This sub-study did not demonstrate any significant effect of dolutegravir with rilpivirine on FRAX scores. This was not surprising because many of the 12 input parameters (e.g. age, BMI, alcohol intake, smoking status, medical history) needed to calculate FRAX scores did not change in this relatively short 48-week study.
The protocol attempted to limit the effect of factors that affect bone density by permitting only stable testosterone or female hormone replacement therapy given for at least 6 months before baseline with no intention to stop during the study; excluding participants with male hypogonadism, uncontrolled thyroid disease, vitamin D deficiency, or severe osteoporosis at baseline; and prohibiting osteoporosis medications (e.g. bisphosphonates). These eligibility criteria prohibited recruitment of some SWORD study participants to the sub-study. We acknowledge the limitations of this bone sub-study. Enrolment of 102 participants provided adequate statistical power for the comparison of change in total hip BMD (as areal density) but not for all categories in the various subgroup analyses. Further, the sub-study was limited by the use of only one time point after baseline (Week 48). Additional assessments may have provided a more detailed analysis of the treatment differences. However, both parent studies and this sub-study are ongoing, with subsequent analyses at weeks 100 and 148 after all participants have been switched to dolutegravir with rilpivirine. Participants in the parent studies randomized to current ART at baseline switched to dolutegravir with rilpivirine at Week 52 if virologically suppressed at Week 48.
No adverse events were considered attributable to the DXA scan procedure; however, there were treatment-related adverse events reported in the parent SWORD-1 and SWORD-2 studies [21]. Over 70% of participants in each treatment group reported adverse events (dolutegravir with rilpivirine, 77%; current ART, 71%); however, adverse events leading to withdrawal were low for both treatment groups (dolutegravir with rilpivirine, 3%; current ART, <1%), indicating that the regimens were well tolerated. Other studies involving switches to regimens containing rilpivirine (GS-US-366-1160 [24] and SPIRIT [25]) or dolutegravir (STRIIVING [26]) have also reported that patients in the current ART groups experienced fewer overall adverse events or discontinuations because of adverse events compared with the switch groups. However, there may be an inherent bias toward the current ART group in switch studies because of the number of years of experience participants had with the previous regimen (GS-US-366-1160, not reported; SPIRIT, 2.8 years; STRIIVING, >4 years; SWORD-1 and SWORD-2, ∼4 years). In these trials, patients in the current ART groups experienced fewer adverse events compared with the switch groups (GS-US-366-1160, 74 versus 80%; SPIRIT, only reported percentages for grades 3 and 4 adverse events; STRIIVING, 47 versus 66%; SWORD-1 and SWORD-2, 71 versus 77%; respectively) and fewer discontinuations because of adverse events (GS-US-366-1160, 2 versus 3%; SPIRIT, 0 versus <1%; STRIIVING, 0 versus 4%; SWORD-1 and SWORD-2, < 1 versus 3%; respectively). Overall, discontinuations because of adverse events were low in the switch groups, which is consistent with increases in treatment satisfaction in the switch group compared with the current ART group in both the STRIIVING and SWORD-1 and SWORD-2 trials.
The little change observed in hip and lumbar spine BMD in participants in the current ART group was expected because the detrimental effect of certain ART regimens on BMD has been reported to slightly decrease [24,27] and stabilize after 1 or 2 years [4]. Participants in the current ART group were receiving tenofovir-containing regimens for at least 6 months before randomization and then another 48 weeks before being switched to dolutegravir with rilpivirine. The observed changes from baseline to Week 48 in total hip and lumbar spine BMD (expressed as areal density, T scores, and Z scores), together with the reduction in bone turnover markers, provide evidence that the switch from a three-drug or four-drug ART regimen containing tenofovir disoproxil fumarate to dolutegravir with rilpivirine was associated with bone health maintenance at minimum, and possibly an improvement through 48 weeks. Together, the BMD and bone marker data from the sub-study indicate that switching to the NRTI-sparing 2DR, dolutegravir with rilpivirine, limits the deleterious effect of ART on BMD and improves markers of bone health, while maintaining viral suppression in patients living with HIV-1 infection.
|
|
| |
| |
|
|
|