| |
Genital inflammation undermines the effectiveness of tenofovir gel in preventing HIV acquisition in women
|
| |
| |
Download the PDF here
"In summary, the combination of gel adherence and genital inflammation differentiated women who were protected by topical tenofovir from those who were not. This was pronounced in participants who did not have genital inflammation but were highly adherent to gel; they experienced protection levels as high as 75%. However, those with genital inflammation who were adherent to gel had no protection, underscoring the unlikelihood of any protective effect in the 'inflammation/adherent' group. Inflammation is a major risk factor for HIV acquisition; reducing genital inflammation through treating its root causes or using anti-inflammatory agents might further optimize PrEP for women. Genital inflammation should be investigated as a potential effect modifier in trials for new PrEP products."
Nature Medicine Feb 25 2018 - Lyle R McKinnon1-3,9, Lenine J Liebenberg1,3,9, Nonhlanhla Yende-Zuma1, Derseree Archary1,3, Sinaye Ngcapu1,3, Aida Sivro1-3, Nico Nagelkerke2, Jose Gerardo Garcia Lerma4, Angela D Kashuba5, Lindi Masson1,6, Leila E Mansoor1, Quarraisha Abdool Karim1,7, Salim S Abdool Karim1,7 & Jo-Ann S Passmore1,6,8
Abstract
Several clinical trials have demonstrated that antiretroviral (ARV) drugs taken as pre-exposure prophylaxis (PrEP) can prevent HIV infection1, with the magnitude of protection ranging from -49 to 86% (refs. 2,3,4,5,6,7,8,9,10,11). Although these divergent outcomes are thought to be due primarily to differences in product adherence12, biological factors likely contribute13. Despite selective recruitment of higher-risk participants for prevention trials, HIV risk is heterogeneous even within higher-risk groups14,15,16. To determine whether this heterogeneity could influence patient outcomes following PrEP, we undertook a post hoc prospective analysis of results from the CAPRISA 004 trial for 1% tenofovir gel (n = 774 patients), one of the first trials to demonstrate protection against HIV infection. Concentrations of nine proinflammatory cytokines were measured in cervicovaginal lavages at >2,000 visits, and a graduated cytokine score was used to define genital inflammation.
In women without genital inflammation, tenofovir was 57% protective against HIV (95% confidence interval (CI): 7-80%) but was 3% protective (95% CI: -104-54%) if genital inflammation was present. Among women who highly adhered to the gel, tenofovir protection was 75% (95% CI: 25-92%) in women without inflammation compared to -10% (95% CI: -184-57%) in women with inflammation. Immunological predictors of HIV risk may modify the effectiveness of tools for HIV prevention; reducing genital inflammation in women may augment HIV prevention efforts.
The overall efficacy of tenofovir gel in this study was 34% (95% CI: -11-61%). Stratifying participants according to genital inflammation clearly segregated efficacy estimates: women in the group with elevated levels of ≥3 cytokines had a tenofovir efficacy of 3% (95% CI: -104-54%; P = 0.936), whereas a tenofovir efficacy of 57% was observed in the group with elevated levels of <3 cytokines (95% CI: 7-80%; P = 0.033) (Fig. 1a). Tenofovir efficacy was 11%, -8%, -147%, and -37% in the groups with elevated levels of ≥4, ≥5, ≥6, and ≥7 cytokines, respectively (all P > 0.1). In contrast, tenofovir efficacy ranged from 34-56% in the corresponding no inflammation groups, and these comparisons were statistically significant (P < 0.05) for the groups with elevated levels of ≥3, ≥5, and ≥6 of the 9 cytokines. ........ Interestingly, HSV-2 positivity mattered more for HIV acquisition if inflammation was not present; this is in line with findings that HSV-2 seroprevalence is not associated with an increased concentration of inflammatory cytokines in cervicovaginal fluid25. Conversely, a high number of sex acts was associated with HIV only in women with genital inflammation.....Tenofovir efficacy was attenuated in women who used the gel infrequently (<50% adherence) irrespective of their inflammation status (25% and 15% efficacy, P = 0.781 and P = 0.656, respectively).....In the strata defined with low adherence, genital inflammation status was the major predictor of HIV acquisition risk (Fig. 1b, solid lines), and there was little evidence of tenofovir-mediated protection.....However, in those with high adherence to tenofovir (Fig. 1c), protection afforded by the gel was restricted to the no inflammation group.....Similar results were obtained for all cytokine scores (≥4, ≥5, ≥6, and ≥7 elevated cytokines; data not shown). These data provide compelling evidence that women without genital tract inflammation largely account for the protective effect of tenofovir gel adherence that was observed in the CAPRISA 004 trial3.
The FGT mucosa typically provides an effective barrier against HIV infection, as reflected by the low per-coital rates of male-to-female HIV transmission in epidemiological studies27,28. Genital inflammation may decrease natural host defenses against HIV, with the corollary being that it is more difficult to use antiviral agents, such as tenofovir, to protect individuals with inflammation against HIV infection. We have previously described reduced levels of key mucosal barrier proteins and increased numbers of cervical CD4+ T cells, the key targets of HIV, in women with cytokine profiles similar to those used in our inflammation scoring29, a finding supported by other studies30,31. This barrier susceptibility hypothesis is corroborated by recent data from CAPRISA 004 showing that women with genital inflammation who were treated with tenofovir had viruses with lower replicative fitness crossing the mucosal barrier to establish HIV infection32. Cellular activation may further increase intracellular dNTP pools and compete with the ability of tenofovir diphosphate to block HIV reverse transcriptase and prevent infection33. Understanding these mechanisms will be critical in designing more effective PrEP strategies, particularly for women.
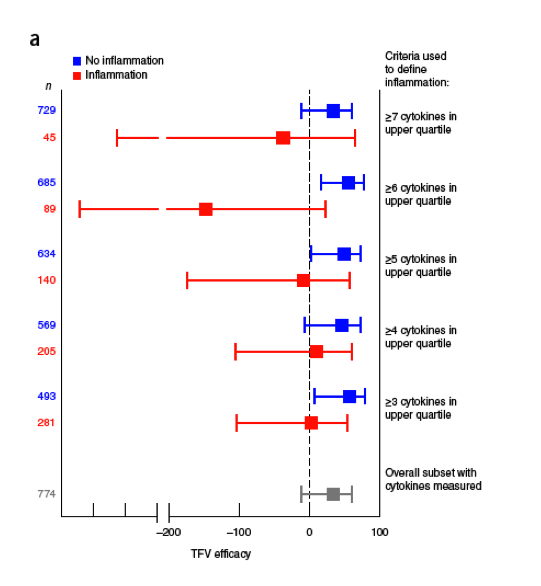
Figure 1 Tenofovir efficacy in groups stratified according to level of inflammation, defined as a specified number of cytokines detected at elevated concentrations in FGT secretions (n = 774 women). (a) Tenofovir efficacy estimates (red boxes) and 95% CIs (whiskers) for groups meeting the indicated cut-offs for inflammation. Data for those falling below the indicated cut-off are represented with blue boxes and whiskers.
Tenofovir efficacy is measured along the x axis, and the dotted black line indicates 0% efficacy. The gray box and whiskers show the overall tenofovir efficacy for all participants included in this analysis. (b,c) Kaplan-Meier survival plots showing the probability of seroconversion in participants stratified according to gel adherence (<50% (b) and ≥50% (c)). Lines indicate data for the tenofovir (TFV) arm of the study, with (solid black) or without (dashed black) genital inflammation, and the placebo arm of the study, with (solid red) or without (dashed red) genital inflammation. Genital inflammation in this analysis was defined as elevated levels of ≥3 of the 9 cytokines. The number of HIV infections and the number of participants at risk for infection in each stratum and at each indicated time point are shown below the graphs (for example, '0/60' indicates there were 0 HIV infections and 60 participants at risk for infection). For statistical
analysis, a z-test was used to compare IRRs between the two study arms. All statistical tests were two-sided and unadjusted for multiple comparisons.
Main
HIV acquisition risk varies widely within a population and is dependent on behavioral and biological factors. Younger women (<25 years of age), for example, experience higher HIV incidence, likely owing to a combination of types and frequencies of sexual partnerships and biological factors, such as genital inflammation17,18. PrEP effectiveness was lowest in women <25 years of age in the vaginal and oral interventions to control the epidemic (VOICE) and dapivirine ring trials; this subgroup was least adherent to PrEP and did not experience substantial protection2,9. The route of HIV exposure may also be important as evidenced by the observation of better oral PrEP protection in men who have sex with men (MSM) under conditions of high adherence10,11 despite higher per-coital acquisition during anal sex than during vaginal sex19. Further, under conditions of lower adherence, protection was still evident in MSM (pre-exposure prophylaxis initiative (iPrEx) trial) but not in women (VOICE trial)2,4. Mucosal tissue penetrance and pharmacokinetics may explain some of these differences; for example, active tenofovir levels in colorectal tissue reach concentrations ten times higher than those in the female genital tract (FGT)20,21.
Protection provided by products that are partially effective may not be equal across groups that are stratified by HIV risk. Protection in the RV144 vaccine trial was higher in individuals at low and medium risk for HIV but negligible in those at the highest risk22. In the iPrEx open-label extension study in MSM, the 'number needed to treat' with PrEP to prevent one infection differed significantly among risk-defined subgroups23. Conversely, in the Partners PrEP trial, participants who consistently used PrEP were protected regardless of their risk profile, suggesting that high adherence and/or effectiveness may overcome differences in susceptibility to infection24.
Case-control analyses of the three trials that have tested topical tenofovir in women (Centre for the AIDS Programme of Research in South Africa (CAPRISA) 004, VOICE, and FACTS001) showed that protection against HIV ranged from 50-60% if product adherence was high2,3,8. These data suggest that adherence alone might not fully explain the incomplete efficacy of this product. Here, we evaluate how biological susceptibility, defined as inflammation in the FGT18, altered the protective efficacy of tenofovir gel.
We carried out a prospective cohort analysis of all available mucosal specimens obtained prior to HIV infection from participants in CAPRISA 004 (n = 774 women sampled at 2,139 visits). Genital inflammation, if defined as elevated levels of ≥3 of 9 examined proinflammatory cytokines (interleukin (IL)-1α, IL-1β, IL-6, tumor necrosis factor (TNF)-α, IL-8, C-X-C motif chemokine 10 (CXCL10; also known as IP-10), monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-1α, and MIP-1β), with 'elevated' defined as a value in the upper quartile of the distribution of the examined cytokine concentrations, was present in 281 women; 204, 140, 90, and 45 women had genital inflammation if defined as elevated levels of ≥4, ≥5, ≥6, and ≥7 of these cytokines, respectively. Women who did not meet the criteria for inflammation were automatically placed in the 'no inflammation' comparator group, so that a total of n = 774 women were included in all analyses. We carried out a Cox regression analysis to determine whether the link between inflammation and HIV risk was dependent on the definition of inflammation that was used. Each definition of genital inflammation was associated with increased HIV risk after adjustment for placement in the tenofovir or placebo study arm (specifically, adjusted hazard ratio (aHR) = 1.86 for elevated levels of ≥3 cytokines (95% CI: 1.11-3.10); aHR = 1.90 for ≥4 cytokines elevated (95% CI: 1.12-3.22); aHR = 2.38 for ≥5 cytokines elevated (95% CI: 1.37-4.15); aHR = 2.99 for ≥6 cytokines elevated (95% CI: 1.64-5.45); and aHR = 3.42 for ≥7 cytokines elevated (95% CI: 1.62-7.23); all P < 0.05). Although the estimates of HIV effect for the groups with elevated levels of ≥3 and ≥4 cytokines were similar, after concentrations of ≥4 of the 9 cytokines were elevated, a step-wise increase in HIV risk of approximately 50% was observed for each subsequent number of elevated cytokines. These inflammation-defined strata were used for the subsequent comparisons of tenofovir efficacy.
We next determined whether tenofovir gel was protective against HIV infection on the basis of the presence or absence of genital inflammation (Table 1). HIV incidence in the study was 3.9 per 100 person-years (95% CI: 2.5-5.8), which was slightly lower than that in the main trial. In all inflammation-defined strata, the lowest HIV incidence rates were observed in women without inflammation who were randomized to tenofovir. In women with elevated levels of ≥3 cytokines, HIV incidence was 6.8 per 100 person-years (95% CI: 3.8-11.1) in the tenofovir arm as compared to 7.0 (95% CI: 3.7-11.9) in the placebo arm. In contrast, in women with elevated levels of <3 cytokines, HIV incidence in the tenofovir and placebo arms was 2.3 (95% CI: 1.0-4.4) and 5.4 (95% CI: 3.4-8.2), respectively. Similar results were obtained when additional numbers of cytokines were elevated; in the strata defined as elevated levels of ≥5, ≥6, and ≥7 cytokines, HIV incidence was higher in women with inflammation randomized to tenofovir than in those randomized to placebo (Table 1).
The overall efficacy of tenofovir gel in this study was 34% (95% CI: -11-61%). Stratifying participants according to genital inflammation clearly segregated efficacy estimates: women in the group with elevated levels of ≥3 cytokines had a tenofovir efficacy of 3% (95% CI: -104-54%; P = 0.936), whereas a tenofovir efficacy of 57% was observed in the group with elevated levels of <3 cytokines (95% CI: 7-80%; P = 0.033) (Fig. 1a). Tenofovir efficacy was 11%, -8%, -147%, and -37% in the groups with elevated levels of ≥4, ≥5, ≥6, and ≥7 cytokines, respectively (all P > 0.1). In contrast, tenofovir efficacy ranged from 34-56% in the corresponding no inflammation groups, and these comparisons were statistically significant (P < 0.05) for the groups with elevated levels of ≥3, ≥5, and ≥6 of the 9 cytokines. Similar results were obtained in multivariate Cox proportional-hazards regression analyses after adjustments for age, study site, herpes simplex virus (HSV)-2 serostatus, history of sexually transmitted infections (STIs), number of sex acts and sexual partners, and condom and injectable contraception use (Table 2). Interestingly, HSV-2 positivity mattered more for HIV acquisition if inflammation was not present; this is in line with findings that HSV-2 seroprevalence is not associated with an increased concentration of inflammatory cytokines in cervicovaginal fluid25.Conversely, a high number of sex acts was associated with HIV only in women with genital inflammation. Considering that gel dosing was per-coital, it is difficult to disaggregate effects of sex and exposure to tenofovir gel in these analyses. Nevertheless, these data confirm that FGT inflammation predicted the efficacy of tenofovir gel in women.
We further tested for interaction between genital inflammation and study-arm membership in a Cox regression analysis with time-varying covariates while taking repeated measures of genital inflammation into account. In a model that incorporated genital inflammation (defined as elevated levels of ≥3 of the 9 cytokines), study arm, and an interaction term between inflammation and study arm, a significant interaction between genital inflammation and study arm was observed (P = 0.028). Similar findings were obtained when genital inflammation was defined as elevated levels of ≥4 and ≥5 of the 9 cytokines, although these were not statistically significant (P = 0.127 and 0.11, respectively). Similar results were obtained in models that included adjustments for potential confounders. These findings support the conclusion that genital inflammation attenuated the efficacy of tenofovir gel.
Previous analyses of results from the CAPRISA 004 trial demonstrated a dose-dependent relationship between gel adherence, measured as the percentage of sex acts for which two gel doses were used, and protection from HIV infection26. We hypothesized that the combination of having no inflammation and high adherence would provide the best protection against HIV infection, and that high levels of inflammation might supersede the protective effects conferred by adherence. Indeed, tenofovir gel-mediated protection was highest in women without genital inflammation who used the gel in ≥50% of sex acts (Supplementary Table 1), and efficacy was 75% (95% CI 25-92%, P = 0.014; <3 elevated cytokines). In comparison, tenofovir efficacy was -10% (95% CI -184-57%, P = 0.844) in highly adherent women with genital inflammation (≥3 elevated cytokines). Tenofovir efficacy was attenuated in women who used the gel infrequently (<50% adherence) irrespective of their inflammation status (25% and 15% efficacy, P = 0.781 and P = 0.656, respectively). Similar results were obtained in adjusted models containing the same covariates as described in Table 2. We also obtained similar results in survival analyses. In the strata defined with low adherence, genital inflammation status was the major predictor of HIV acquisition risk (Fig. 1b, solid lines), and there was little evidence of tenofovir-mediated protection. However, in those with high adherence to tenofovir (Fig. 1c), protection afforded by the gel was restricted to the no inflammation group. Similar results were obtained for all cytokine scores (≥4, ≥5, ≥6, and ≥7 elevated cytokines; data not shown). These data provide compelling evidence that women without genital tract inflammation largely account for the protective effect of tenofovir gel adherence that was observed in the CAPRISA 004 trial3.
The FGT mucosa typically provides an effective barrier against HIV infection, as reflected by the low per-coital rates of male-to-female HIV transmission in epidemiological studies27,28. Genital inflammation may decrease natural host defenses against HIV, with the corollary being that it is more difficult to use antiviral agents, such as tenofovir, to protect individuals with inflammation against HIV infection. We have previously described reduced levels of key mucosal barrier proteins and increased numbers of cervical CD4+ T cells, the key targets of HIV, in women with cytokine profiles similar to those used in our inflammation scoring29, a finding supported by other studies30,31. This barrier susceptibility hypothesis is corroborated by recent data from CAPRISA 004 showing that women with genital inflammation who were treated with tenofovir had viruses with lower replicative fitness crossing the mucosal barrier to establish HIV infection32. Cellular activation may further increase intracellular dNTP pools and compete with the ability of tenofovir diphosphate to block HIV reverse transcriptase and prevent infection33. Understanding these mechanisms will be critical in designing more effective PrEP strategies, particularly for women.
Strengths of this study include its longitudinal design and large sample size; it is one of the largest studies of mucosal inflammation in the context of a trial for HIV prevention. Genital inflammation was evaluated at repeated measures in participants randomized to tenofovir or placebo gel, and inclusion of the entire available cohort allowed us to calculate HIV incidence and tenofovir efficacy in subgroups of at-risk individuals. The study tested an a priori hypothesis, and immunological analyses were blinded with extensive quality-control measures put in place to ensure the accuracy of cytokine measurements across multiple sample runs. Although inflammation is difficult to capture using any one measurement, proinflammatory cytokines are believed to be central to this process. Our sensitivity analyses support the notion that inflammation was consistently able to differentiate women protected by tenofovir, irrespective of how many cytokines were elevated.
Our study had some limitations. Specimens were available only at certain study visits and were not available for a subset of individuals, including some who acquired HIV before the first available genital sample was taken. However, in the remaining cohort, we took a median measure of several visits and were therefore better able to classify individuals than what could be done using a single measurement. Our major conclusions were further borne out by a second time-varying analysis, showing a significant interaction between genital inflammation (≥3 elevated cytokines) and study arm in predicting HIV acquisition. For adherence, we relied on self-reported return of used applicators. Data on the mucosal concentration of tenofovir are available for a subset of participants26, but too few for comparisons of gel efficacy. We based our adherence analyses on self-report that the product was used in ≥50% sex acts; although this has clear clinical implications regarding protection, the study is underpowered for further adherence cut-offs and for adherence-inflammation interaction analyses. Despite our large sample size, a relatively small proportion of the cohort had genital inflammation, limiting statistical power to definitively conclude that those with inflammation were not protected by tenofovir gel. Further validation in additional cohorts could increase this sample size, but this has logistical challenges. Finally, we deliberately selected composite cytokine outputs on the basis of prior studies18 to overcome the burden of multiple-test correction for individual cytokine concentrations, and we used multiple elevated cytokine cut-offs to determine the rigor of these definitions of genital inflammation in assessing HIV outcomes.
The causes of inflammation remain unclear. Several groups have shown that bacterial vaginosis (BV) and/or changes in the vaginal microbiome are associated with genital inflammation34,35, including in CAPRISA 004 (unpublished data). We and others have also recently shown that vaginal dysbiosis impairs tenofovir efficacy, perhaps via reducing levels of tenofovir in the mucosa36. Interestingly, the same issue may not apply to oral PrEP, as BV and/or vaginal dysbiosis did not affect PrEP efficacy in the Partners PrEP study37. This could be due to pharmacological differences between oral and topical PrEP. As not all BV and/or dysbiosis results in inflammation38, repeating our inflammation analyses in other PrEP studies will help to understand the generalizability of our findings.
In summary, the combination of gel adherence and genital inflammation differentiated women who were protected by topical tenofovir from those who were not. This was pronounced in participants who did not have genital inflammation but were highly adherent to gel; they experienced protection levels as high as 75%. However, those with genital inflammation who were adherent to gel had no protection, underscoring the unlikelihood of any protective effect in the 'inflammation/adherent' group. Inflammation is a major risk factor for HIV acquisition; reducing genital inflammation through treating its root causes or using anti-inflammatory agents might further optimize PrEP for women. Genital inflammation should be investigated as a potential effect modifier in trials for new PrEP products.
|
|
| |
| |
|
|
|