| |
Survey on Cure Research Among HIV+ presented at NIH Strategies for an HIV Cure Meeting
|
| |
| |
Reported by Jules Levin
NATAP
It appears this effort's goal was to try to identify what HIV+ would like to see in a cure or remission treatment or drug. What is not apparently asked here is if HIV+ understand they will get nothing out of participating in a cure study now because any benefits ultimately found will not help them personally by participating in this study. Upon reading through these findings it appears to me there a number of disconnects between what HIV+ would like from a cure or remission and how these studies are conducted. Of note several times it was mentioned that ART is thought by many HIV+ to be relatively adequately satisfying in the current environment and so a high threshold would have to be met to replace this with a cure or remission, this is explored below in many questions. I think the survey also raises questions about whether potential participants truly understand what they are getting into by participating in the study and what they think they are getting out of it, and don't understand some risks. I'm not sure all the researchers themselves understand some of the risks, in particular the risk for CNS viral escape with even a brief ATI. Jules
WEBCAST: https://videocast.nih.gov/summary.asp?Live=28488&bhcp=1
this presentation begins around 1:03:46 into webcast.

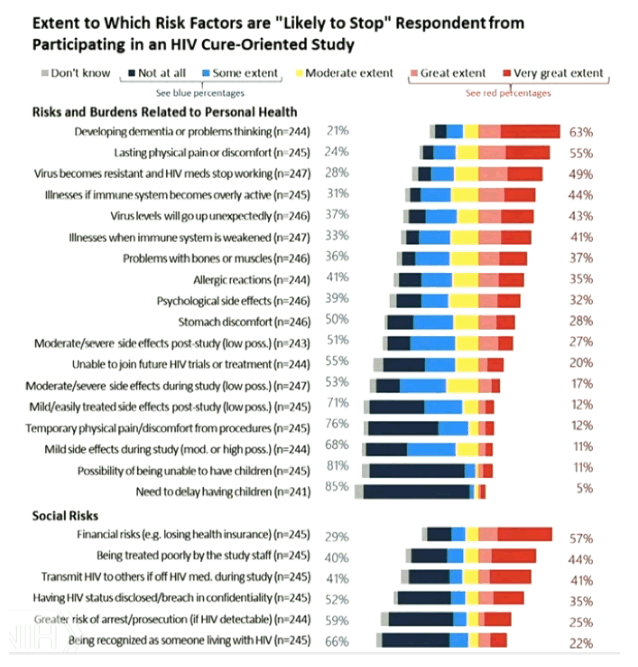
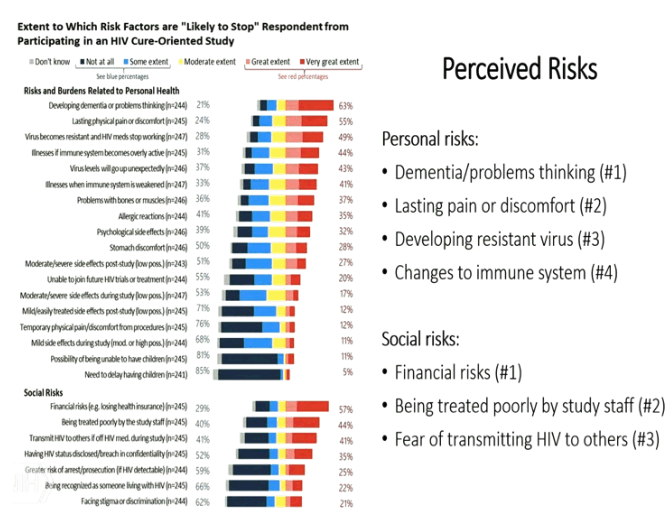
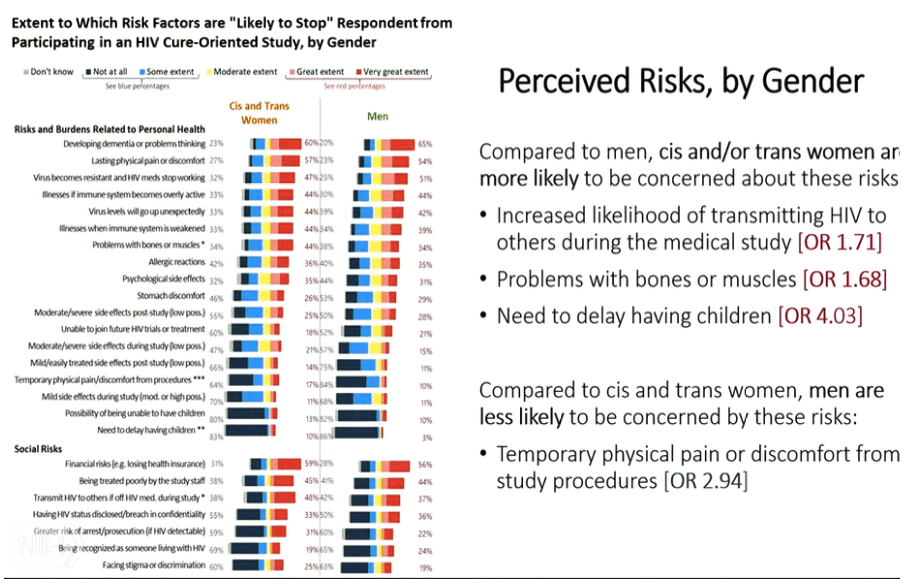
from Jules: I would note the most mentioned concern & depeterrant to participating in cure research is the fear of developing dementia or problem thinking. Ironically the main issue I have been trying to raise is the issue that ATIs can cause CNS viral escape which potentially could cause long term cognitive or neurologic impairment, and this concern is raised in several publications linked to below, and we had a conference call where 2 key neurologists said this potential is possible and the risk has not been researched well, the sliders from that call are attached. But what is interesting is that I am sure there is no informed consent that potential participants in cure studies are informed of this risk nor are they explained this risk and I am certain they have no idea of this risk, plus I don't think most of the researchers themselves pay any attention to this risk nor disclose it nor do they even understand this might be a risk. Of course there is also the risk that an ATI might reseed the brain & CNS reservoirs or cause inflammation.....
ATI and the Central Nervous System (CNS): Introduction - (05/16/18) conference call slides
ART Interruption Associated with HIV in the Brain: "Reseeding CNS during ART Interruption Leading to Intrathecal Immune Activation" - "HIV-1 Viral Escape in Cerebrospinal Fluid of Subjects on Suppressive Antiretroviral Treatment" - (09/18/17)-
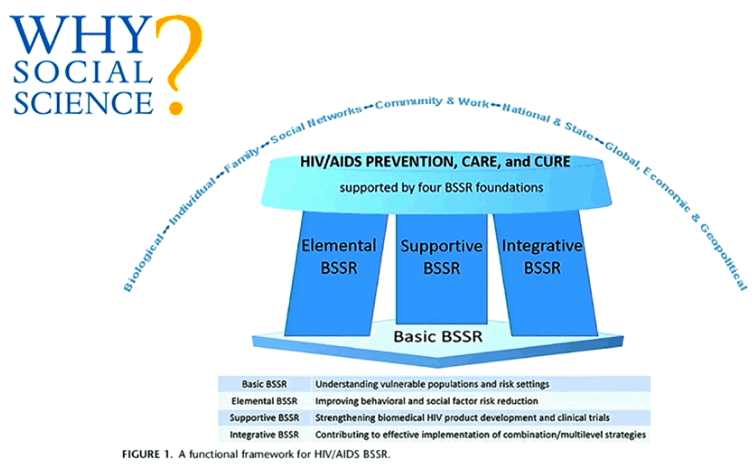
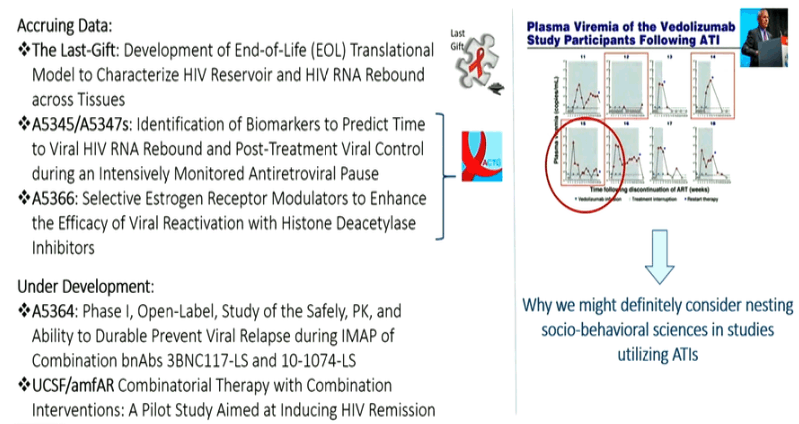
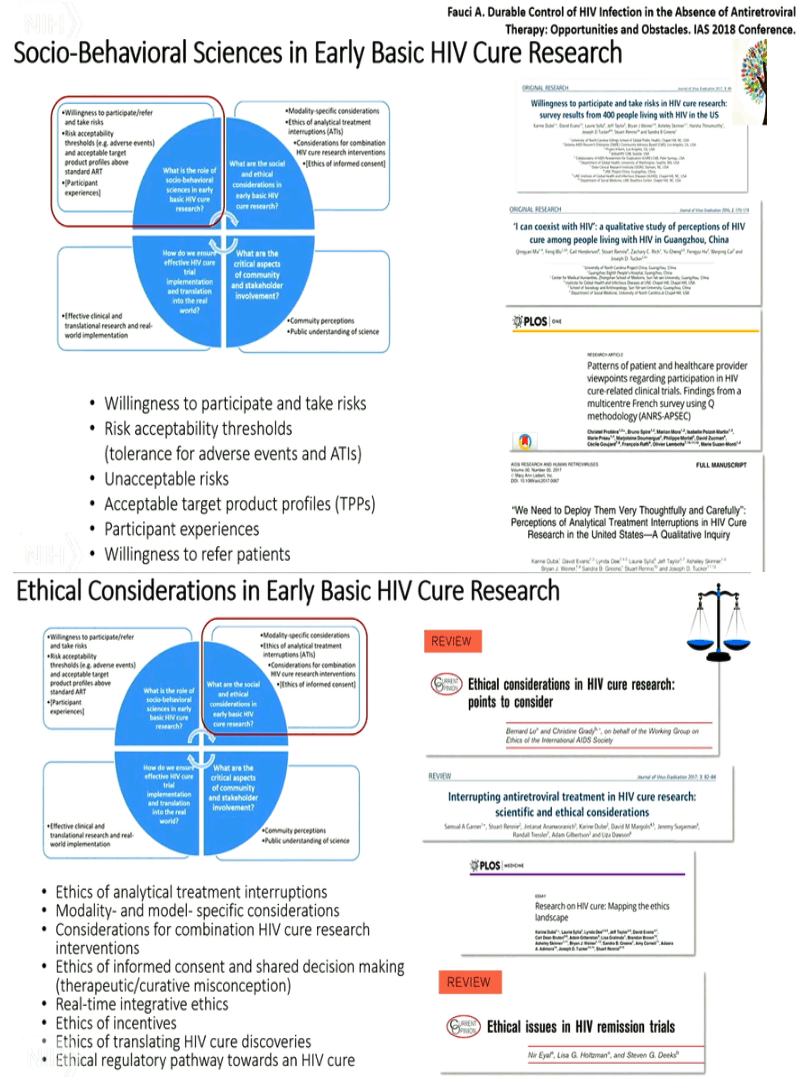
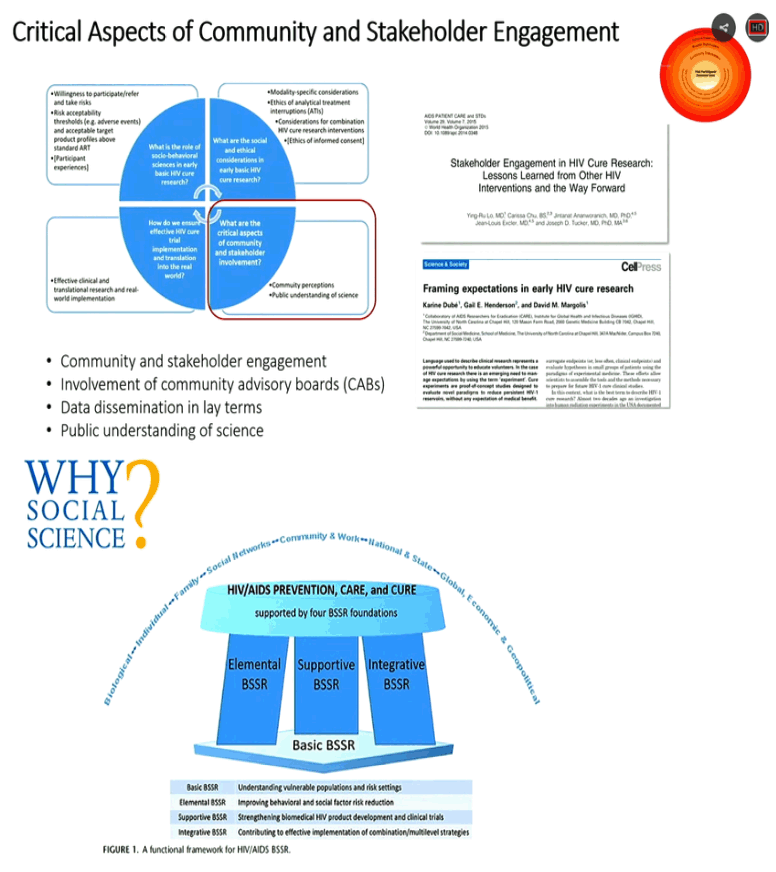
New Cure research studies, ART interruptions: biomarkers for viral rebound after interruptions, search for reservoirs where HIV rebounds from; intensified monitoring, safety concerns; patient consent
Cure Studies/ART Interruption & Brain Affects, Vorinostat - (10/13/17)
HIV cure strategies [interruptions/viral rebound brain/immune affects]: response to ignore the central nervous system at your patients' peril - (12/20/17)
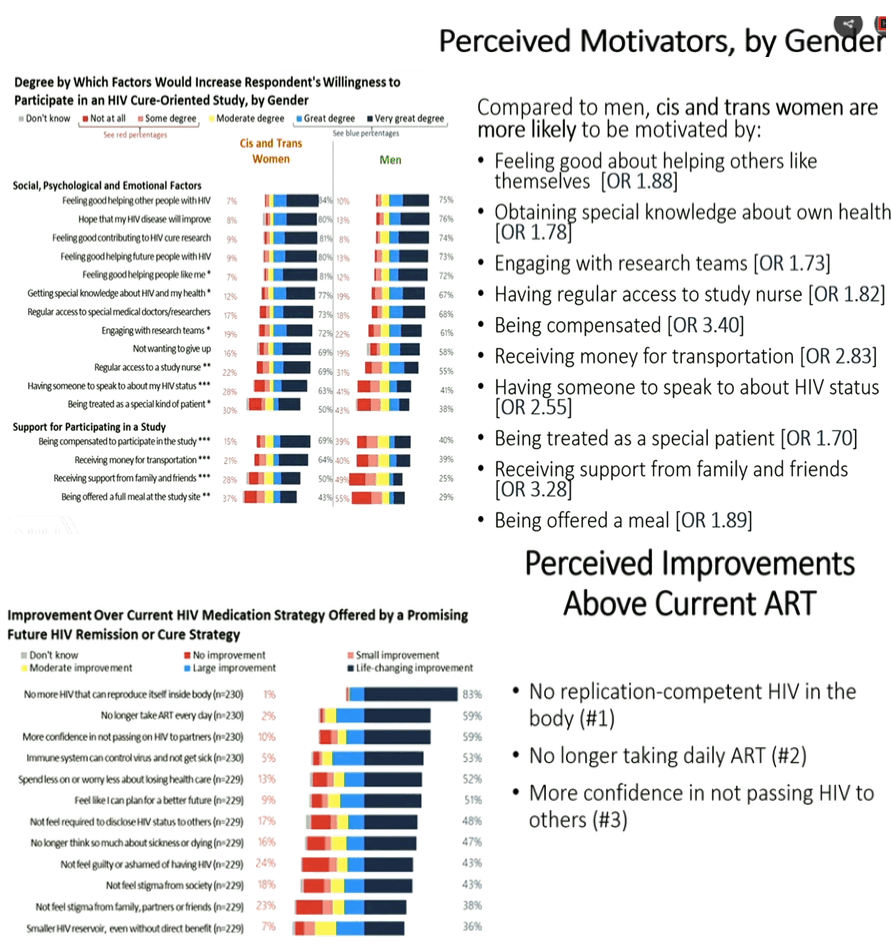
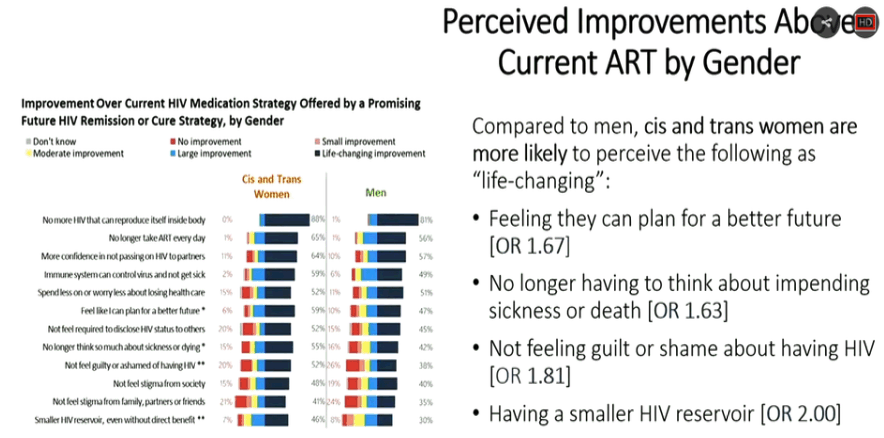
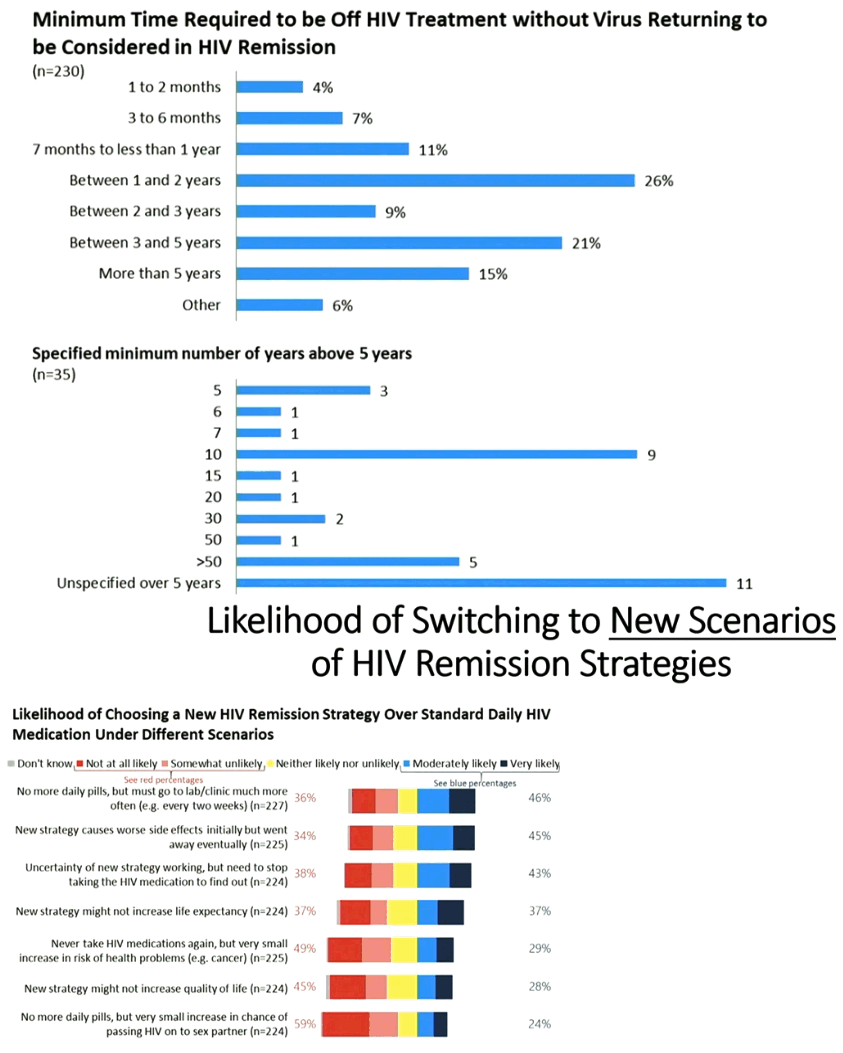
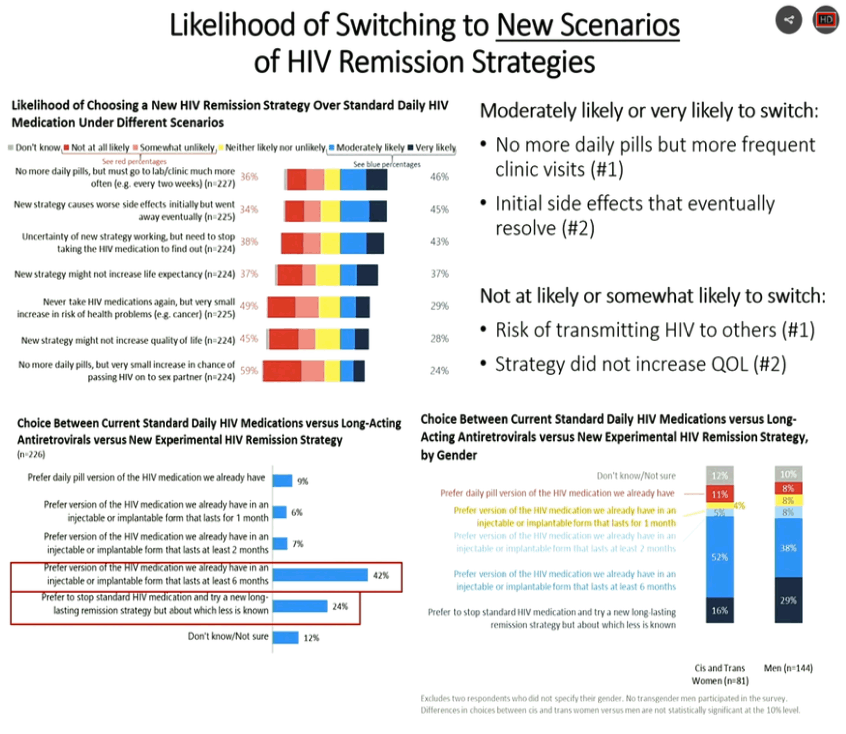


|
|
| |
| |
|
|
|