 |
 |
 |
| |
Tail-phase safety, tolerability and pharmacokinetics of long-acting injectable cabotegravir in HIV-uninfected individuals: HPTN 077 final results
|
| |
| |

HIV Research for Prevention (HIVR4P), October 21-25, 2018, Madrid
Mark Mascolini
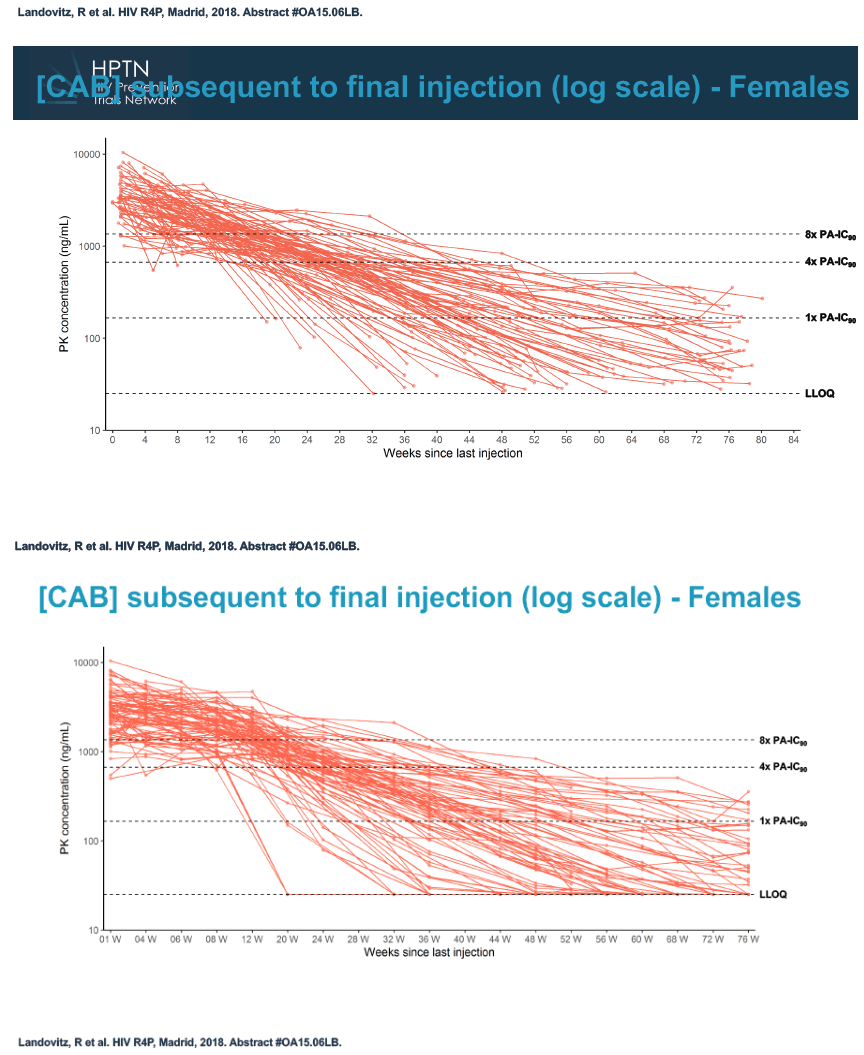
Slowly dwindling cabotegravir levels after a final dose of the long-acting PrEP candidate linger months longer in women than in men, according to results of HPTN 077 [1]. Ongoing studies may help define clinical implications of prolonged cabotegravir exposure in women and men.
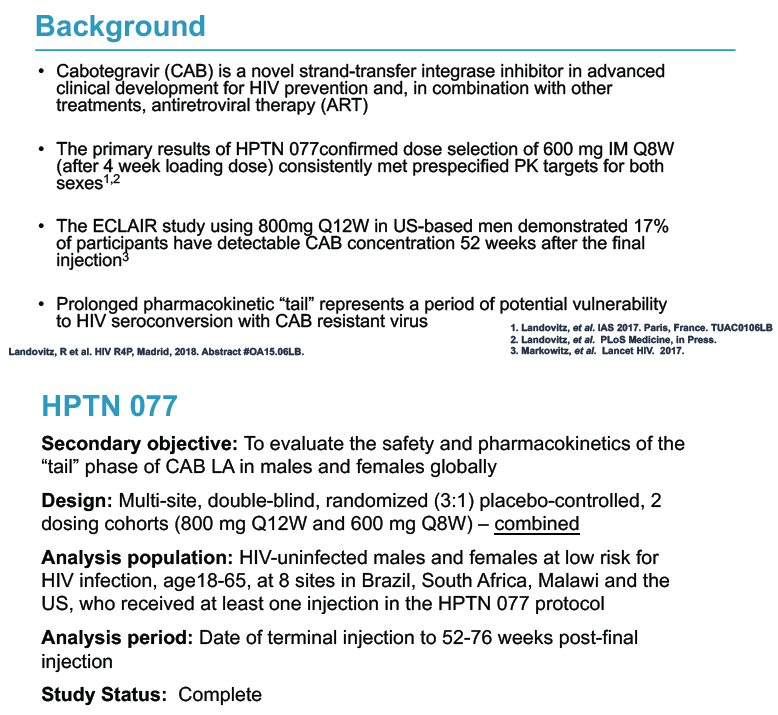
Cabotegravir is an HIV integrase inhibitor in late stages of development (1) with other antiretrovirals for chronic HIV infection and (2) as a sole agent for preexposure prophylaxis (PrEP). The international HPTN 077 trial established that an intramuscular dose of 600 mg every 8 weeks (after a 4-week oral loading dose) met drug level targets for women and men [2]. The ECLAIR study in US men found that 800 mg of cabotegravir every 12 weeks left detectable levels of the integrase inhibitor 52 weeks after the final injection in 17% of participants [3]. Slowly dwindling drug levels after a drug stops--called a pharmacokinetic tail--pose a threat of HIV infection with virus resistant to that drug.
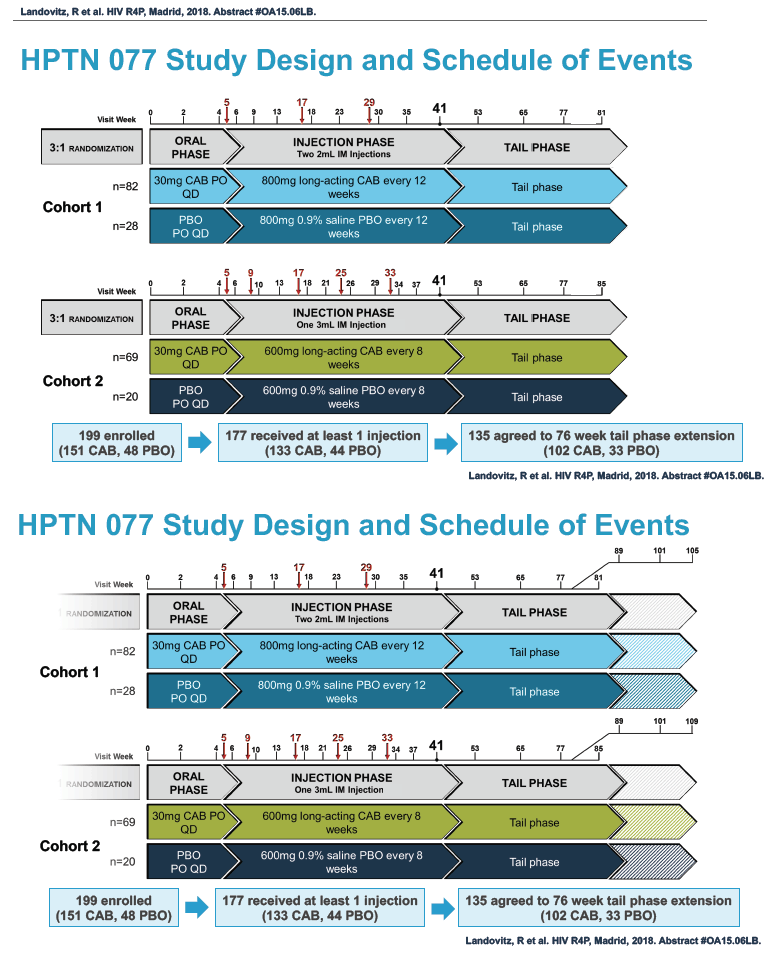
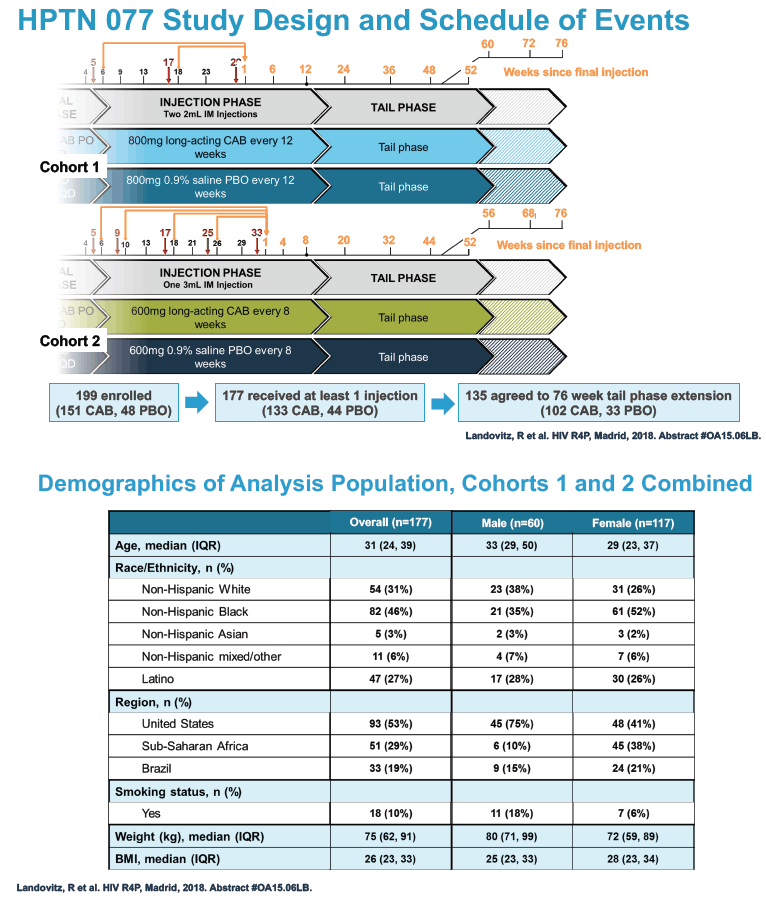
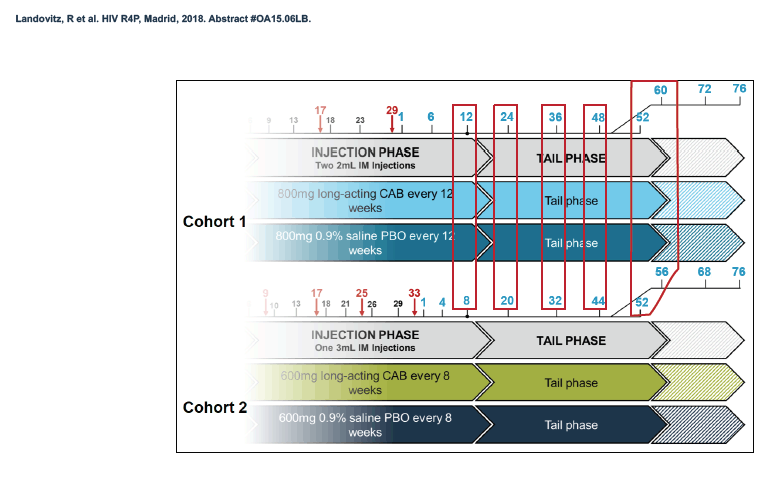
HPTN 077 also aimed to assess the safety and pharmacokinetics of the cabotegravir tail in female and male trial participants. This double-blind, placebo-controlled trial enrolled HIV-negative adults in Brazil, South Africa, Malawi, and the United States and tested two intramuscular cabotegravir doses: 800 mg every 12 weeks and 600 mg every 8 weeks [2]. Among 177 participants who received at least one cabotegravir or placebo injection, 135 agreed to a 76-week tail-phase extension (102 on cabotegravir and 33 on placebo).
In the overall 177-person study group, 117 were women and 60 men. Median age stood at 31 years, 46% were black, 31% white, and 27% Hispanic. About half of the study group, 53%, lived in the United States, 29% in Africa, and 19% in Brazil. Median body mass index measured 28 kg/m2 in women and 25 kg/m2 in men. Respective median weights were 72 kg in women and 80 kg in men. A body mass index of 25 kg/m2 is the threshold for overweight.
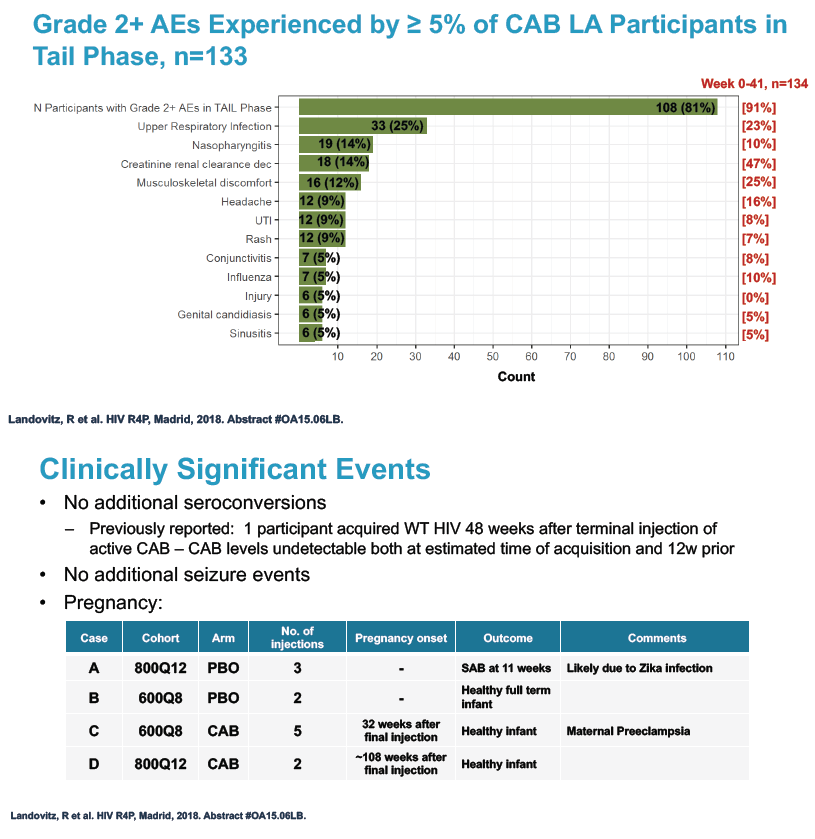
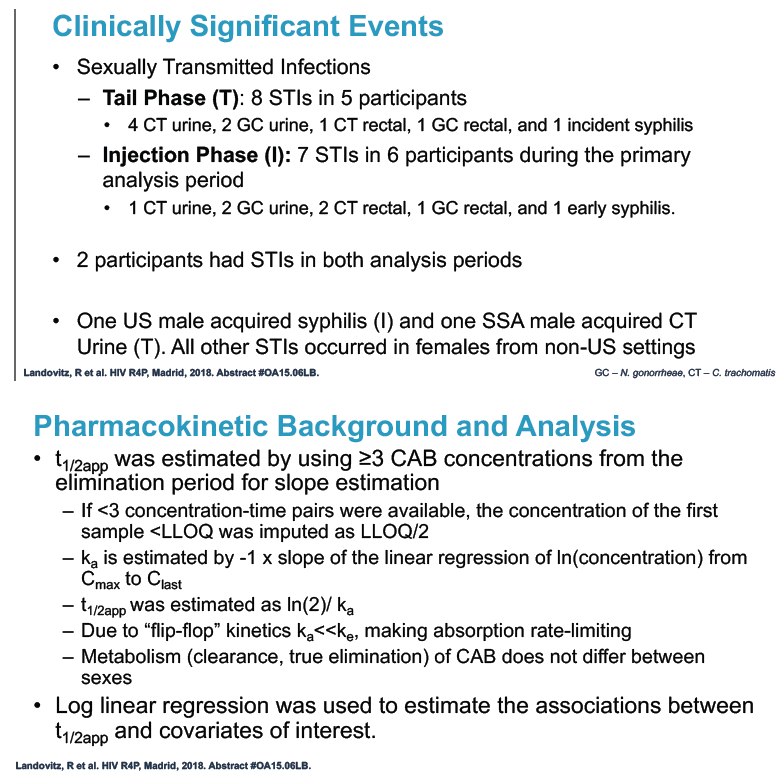
No one became infected with HIV during the tail phase. Among the 133 tail-phase participants, 81% had a grade 2 or greater adverse event, usually upper respiratory infection (25%), nasopharyngitis (14%), and falling creatinine clearance (14%). Clinically significant tail-phase events included 8 sexually transmitted infections in 5 participants.
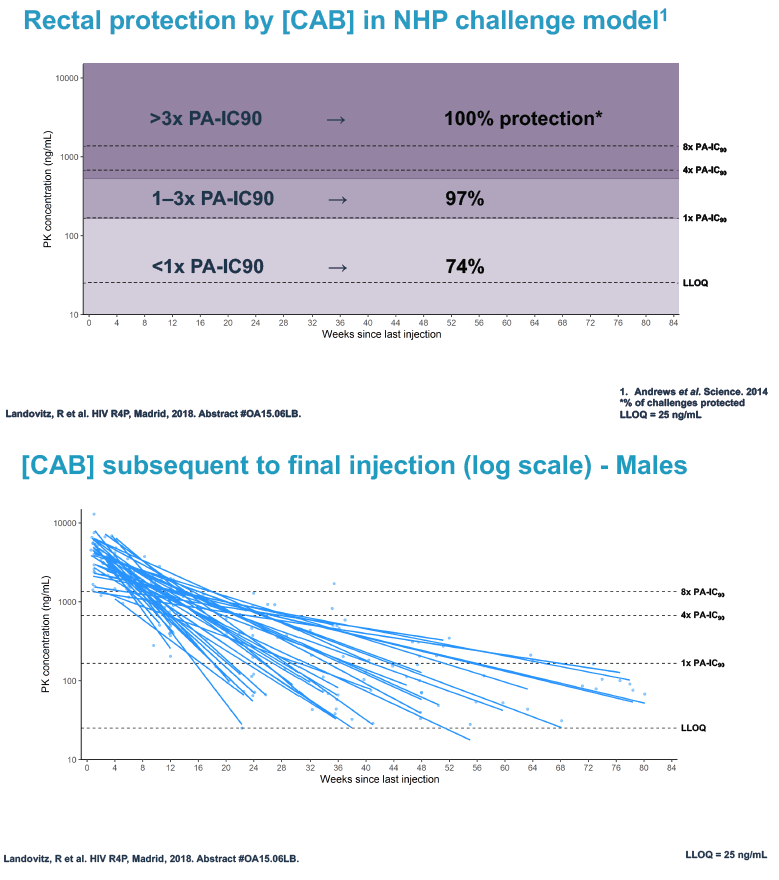
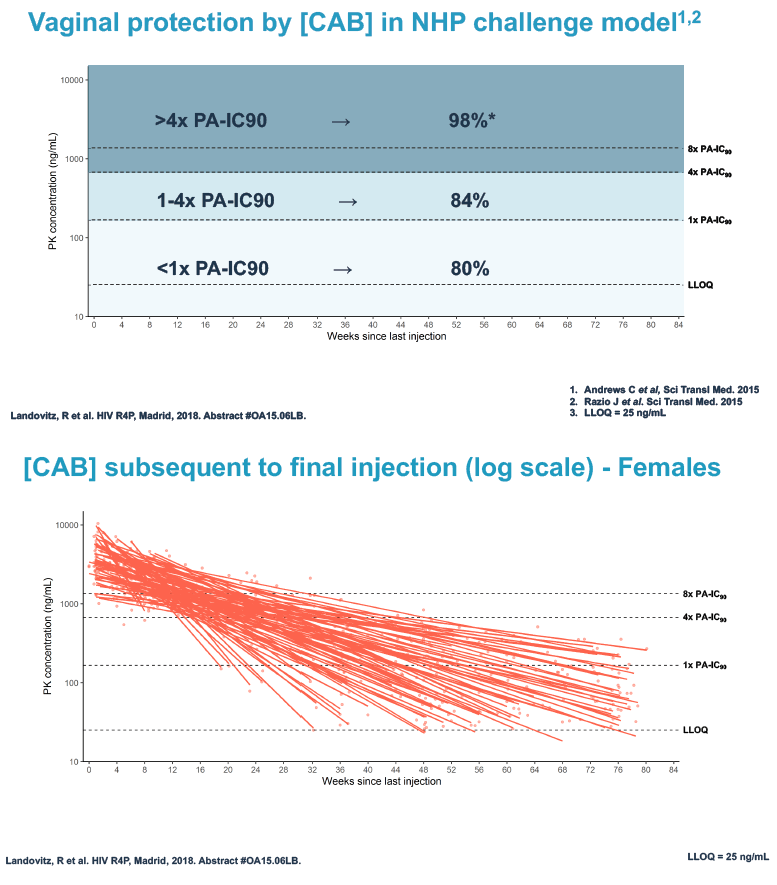
Researchers used 3 or more cabotegravir concentrations from the tail phase to estimate cabotegravir half-life. They used log linear regression analysis to explore associations between variables of interest and cabotegravir half-life. The investigators applied data from monkey studies to determine rectal and vaginal protection by cabotegravir at 3 concentration ranges for males and females:
Rectal protection
1. More than 3 x the protein-adjusted 90% inhibitory concentration (PA-IC90): 100% rectal protection
2. 1 to 3 x the PA-IC90: 97% rectal protection
3. Less than 1 x the PA-IC90: 74% rectal protection
Vaginal protection
1. More than 4 x the PA-IC90: 98% vaginal protection
2. 1 to 4 x the PA-IC90: 84% vaginal protection
3. Less than 1 x the PA-IC90: 80% vaginal protection
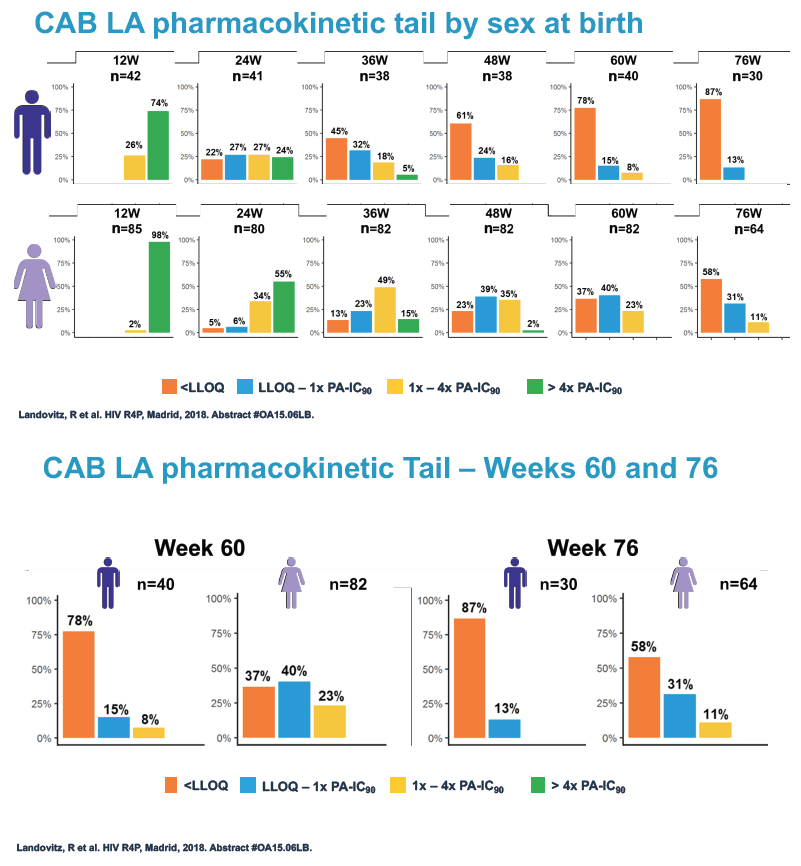
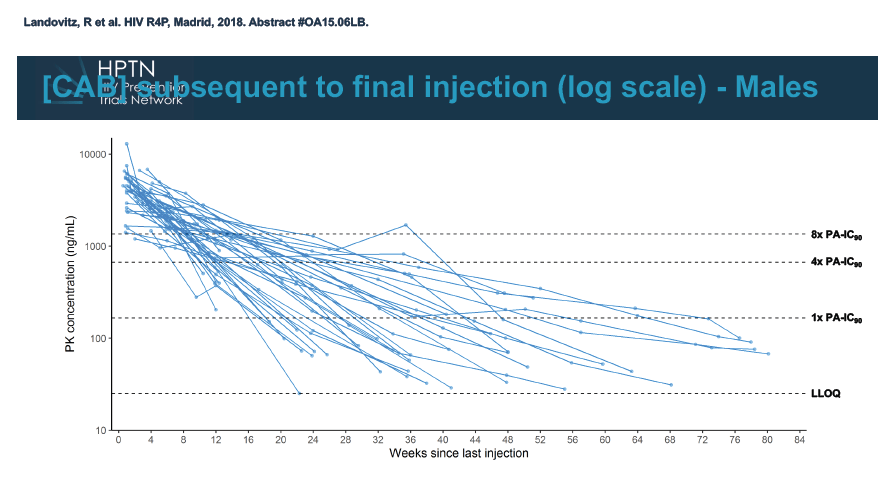
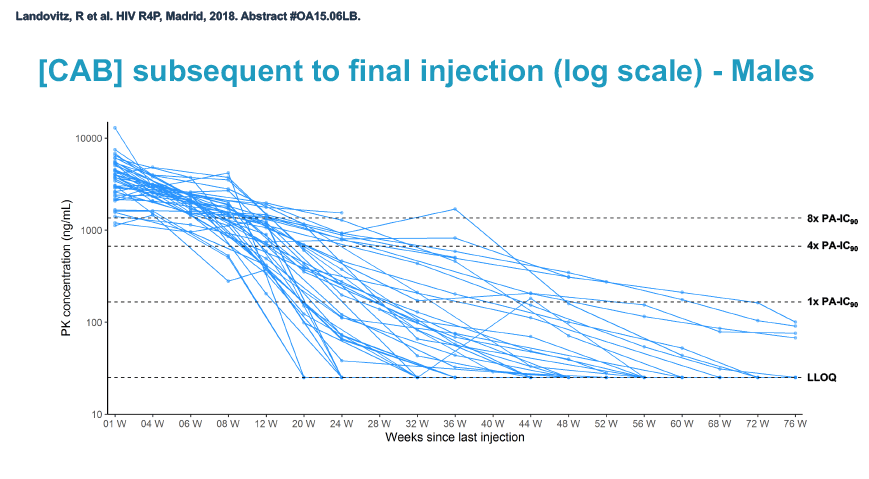
At week 60 of the HPTN-077 tail-phase analysis, 78% of 40 men versus 37% of 82 women had cabotegravir concentrations below the limit of detection. At week 72 of the tail phase, 87% of 30 men versus 58% of 64 women had undetectable cabotegravir. Proportions with a cabotegravir level between the lower limit of detection and 1 x the PA-IC90 were 15% for men versus 40% for women at week 60 and 13% for men versus 31% for women at week 76. Proportions with a cabotegravir level between 1 to 4 x the IC90 were 8% for men versus 23% for women at week 60 and 0% for men versus 11% for women at week 76.
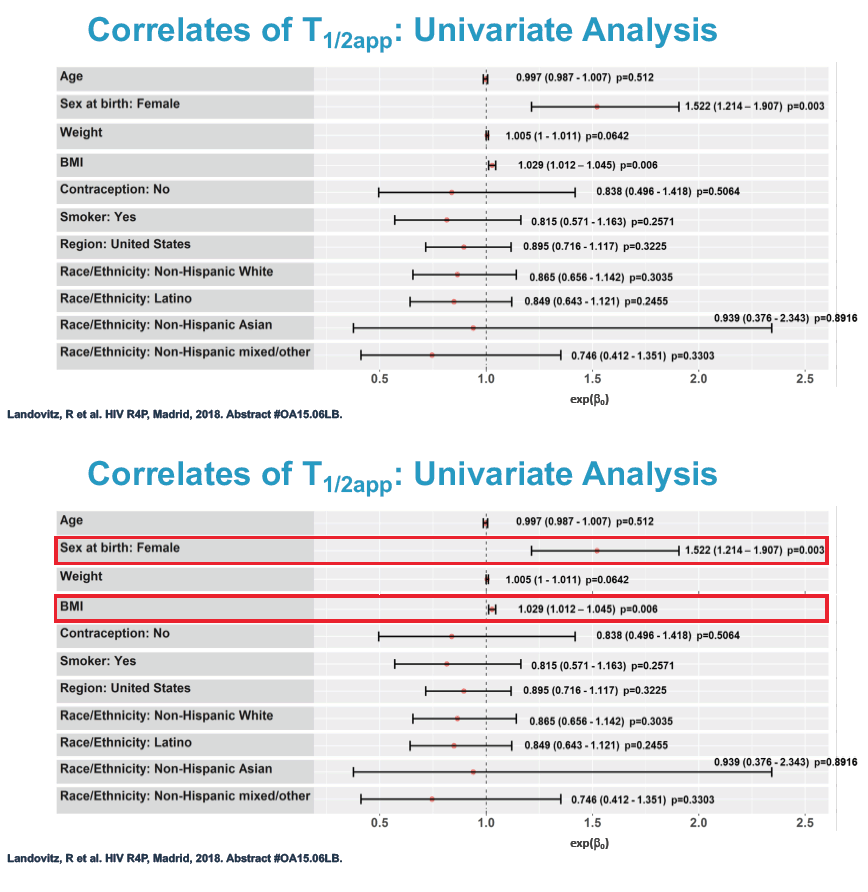
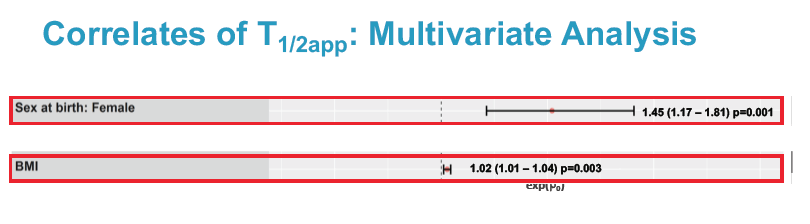
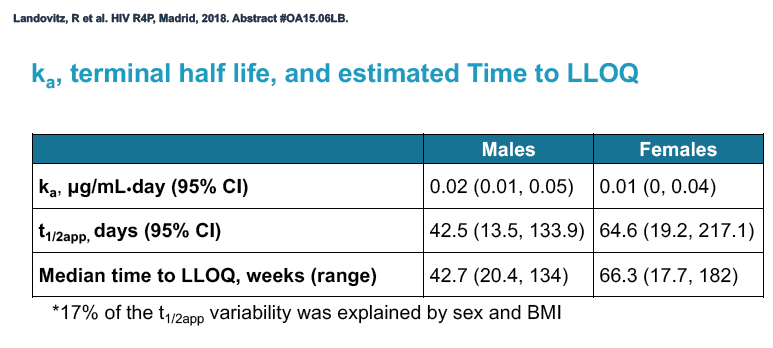
Multivariate linear regression analysis identified 2 factors independently associated with longer cabotegravir half-life: being a woman (exp(beta0) 1.45, 95% confidence interval 1.17 to 1.81, P = 0.001) and greater body mass index (exp(beta0) 1.02, 95% confidence interval 1.01 to 1.04, P = 0.003).
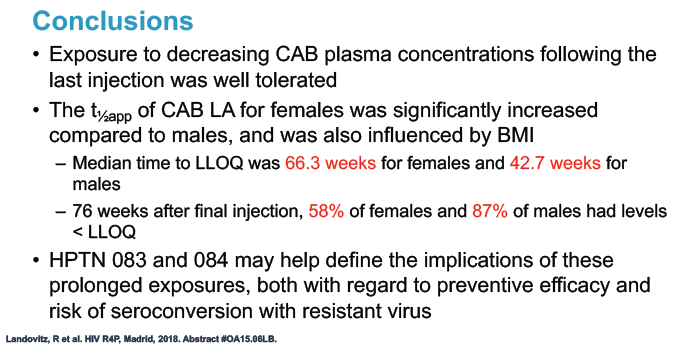
Median time to undetectable cabotegravir in the tail phase stood at 42.7 weeks (range 20.4 to 134) in men and 66.3 weeks (range 17.7 to 182) in women. In other words the cabotegravir tail stretched almost a half-year longer (23.6 weeks) in women than in men.
The HPTN 077 investigators concluded that (1) waning cabotegravir exposure after the final dose is well tolerated and (2) long-acting cabotegravir half-life is significantly longer in women than in men and is also influenced by body mass index.
References
1. Landovitz RJ, Eron JJ, Grinsztein G, et al. Tail-phase safety, tolerability and pharmacokinetics of long-acting injectable cabotegravir in HIV-uninfected individuals: HPTN 077 final results. HIV Research for Prevention (HIVR4P), October 21-25, 2018, Madrid. Abstract OA15.06.
2. Landovitz R, Li S, Grinsztejn B, et al. Safety, tolerability and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected women and men: HPTN 077. IAS 2017. Paris. Abstract TUAC0106LB. http://www.natap.org/2017/IAS/IAS_33.htm
3. Markowitz M, Frank I, Grant RM, et al. Safety and tolerability of long-acting cabotegravir injections in HIV-uninfected men (ECLAIR): a multicentre, double-blind, randomised, placebo-controlled, phase 2a trial. Lancet HIV. 2017;4:e331-e340. |
WEBCAST: http://webcasts.hivr4p.org/console/player/40424?mediaType=slideVideo&&crd_fl=1&ssmsrq=1540469197314&ctms=5000&csmsrq=1315
--------------------------

Reported by Jules Levin
HIV R4P - Madrid, Spain
Wednesday, October 24, 2018
RJ Landovitz, S Li, JJ Eron, B Grinsztejn, H Dawood, AY Liu, M Magnus, MC Hosseinipour, R Panchia, L Cottle, P Richardson, MA Marzinke, SH Eshleman, R Kofron, A Adeyeye, D Burns, AR Rinehart, D Margolis, M McCauley, CW Hendrix

|
| |
|
 |
 |
|
|