 |
 |
 |
| |
Universal test-and-treat improves population viral suppression in rural South Africa
|
| |
| |
22nd International AIDS Conference, Amsterdam, Netherlands, July 23-27, 2018
Mark Mascolini
A universal test-and-treat approach to HIV management improved population viral suppression in the ANRS TasP trial in rural South Africa, more through universal testing than universal treatment [1]. Randomization to immediate testing and treatment did not cut HIV incidence--the new-infection rate.
Universal test-and-treat involves testing everyone at risk for HIV infection and immediately treating those who test positive. The strategy aims to maximize the proportion of people living with HIV who get antiretroviral therapy (ART) and achieve viral suppression--and thus to boost population-level suppression and limit HIV transmission. Mathematical modeling predicts that universal test-and-treat will cut HIV incidence and perhaps eliminate HIV epidemics.
One of 5 international test-and-treat trials, ANRS 12249 TasP is a cluster-randomized trial in the rural Hlabisa subdistrict of South Africa's KwaZulu Natal province. Running from March 2012 through June 2016, the trial enrolled people at least 16 years old. The subdistrict has about 28,000 adult residents and an HIV prevalence around 30%. Only 10% of residents have jobs, and migration is frequent.
HIV counselors visited all local households and counted resident adults. Eligible participants completed a sociodemographic and sexual behavior questionnaire and gave a finger-prick sample to allow HIV testing of dried blood spots. Counselors repeated these home-based tests and surveys every 6 months and recorded deaths and out-migrations. Everyone who tested positive for HIV or reported HIV infection got referred to a local clinic within 45 minutes walking distance. Clinics in control clusters offered ART according to national guidelines; clinics in test-and-treat clusters offered immediate ART regardless of CD4 count or clinical stage.
Four clusters opened in 2012, 6 in 2013, and 12 in 2014. Follow-up continued in all clusters through mid-2016. As previously reported, HIV incidence did not differ significantly between the two trial arms [2]. The current analysis asked whether population viral suppression improved during the course of the trial and whether change in population viral suppression differed between study arms.
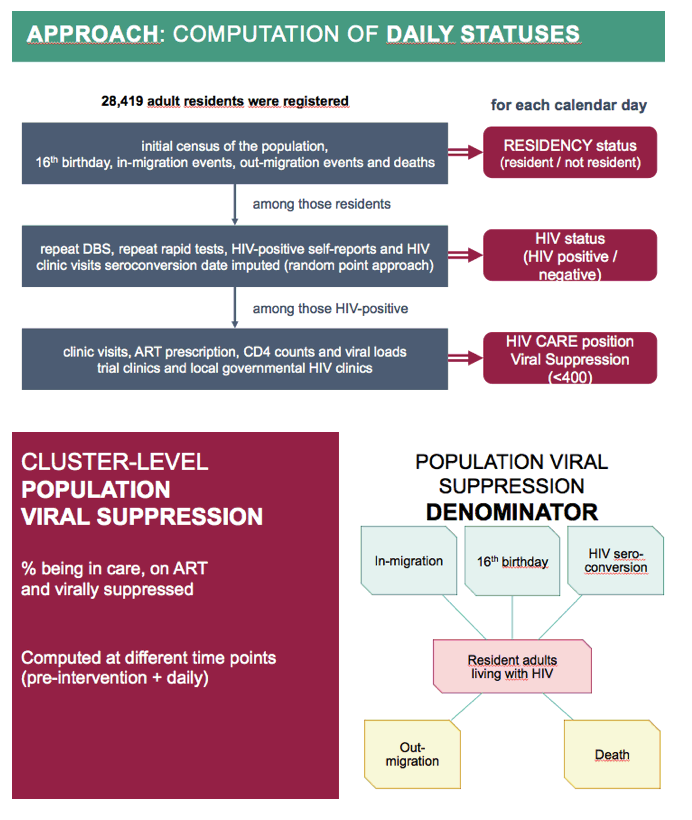
The ANRS team used multiple sources to estimate daily HIV status. They used data on clinic visits, ART prescriptions, and CD4 count to estimate how many people with HIV entered care and started ART, and how many treated people reached a viral load below 400 copies. They defined population viral suppression as the proportion of resident adults with HIV in care, on ART, and with a viral load below 400 copies. The researchers computed population viral suppression before the intervention and per day as soon as they completed the initial population census.
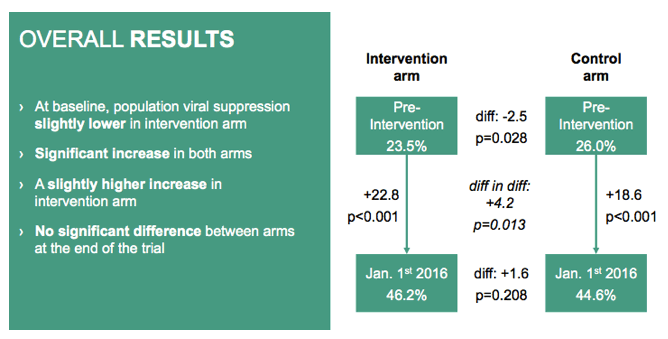
Pre-intervention population viral suppression was slightly but significantly lower in the test-and-treat arm than in the control arm (23.5% versus 26.0%, P = 0.028). Through January 1, 2016, the population viral suppression rate rose significantly to 46.2% in the test-and-treat arm (+22.8%, P < 0.001) and to 44.6% in the control arm (+18.6%, P < 0.001). The 2016 suppression rate did not differ significantly between the test-and-treat arm and the control arm (46.2% and 44.6%, P = 0.208).
The researchers used a mixed linear model to explore relations between population viral suppression and calendar time, time since cluster opening, trial arm, and interaction between arm and time since cluster opening. This analysis adjusted for cluster-level sociodemographic changes. Modeling suggested that time since cluster opening mainly drove the increase in population viral suppression. The analysis indicated that the impact of universal treatment was smaller than the impact of universal testing because of low linkage to care.
The investigators concluded that a universal test-and-treat strategy significantly improved population viral suppression over time in rural South Africa, even though it did not lower HIV incidence more than in the control arm [2]. The ANRS team noted that they did not power the trial to measure whether HIV incidence fell over time within each arm but to compare overall incidence between arms.
References
1. Lamarange J, Diallo MH, McGrath N, et al. Temporal trends of population viral suppression in the context of universal test and treat: results from the ANRS 12249 TasP trial in rural South Africa. AIDS 2018: 22nd International AIDS Conference, Amsterdam, Netherlands, July 23-27, 2018. Abstract TUAC0103.
2. Iwuji CC, Orne-Gliemann J, Larmarange J, et al; ANRS 12249 TasP Study Group. Universal test and treat and the HIV epidemic in rural South Africa: a phase 4, open-label, community cluster randomised trial. Lancet HIV. 2018;5:e116-e125. https://www.ncbi.nlm.nih.gov/pubmed/29199100

Although suboptimal, the UTT strategy implemented in TasP trial improved significantly population viral suppression over time.
As it was mainly due to universal testing rather than universal treatment, it did not induce difference between arms, explaining the null effect observed on cumulative incidence. Unfortunately, the trial was not powered to measure if incidence decreased over time within each arm, but to compare if overall incidence was different between arms.
CLIC
Changes in treatment initiation guidelines alone are not enough to significantly increase population viral suppression if no additional intervention is implemented to improve linkage to care in this rural setting.
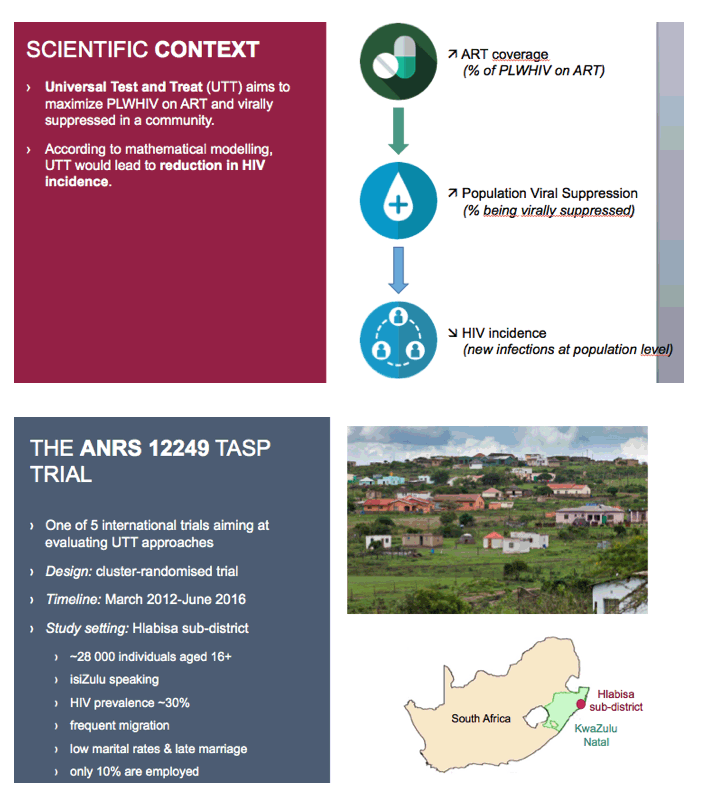
The ANRS 12249 Treatment as Prevention trial is one of 5 internationals trials aiming at evaluating UTT approaches.
It was a phased two-arm cluster-randomised trial implemented between March 2012 and June 2016 in Hlabisa sub-district, northeast KwaZulu-Natal, South Africa, in a rural area with approximately 28,000 isiZulu-speaking resident adults.
Adult HIV prevalence in the sub-district was around 30%. Hlabisa sub-district is characterized by frequent migration, low marital rates, and late marriage. On average only one adult in ten in the trial area is employed.
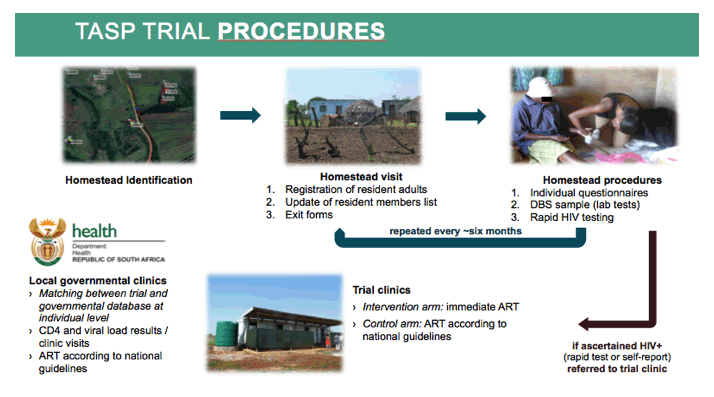
In both trial arms, HIV counsellors visited all local households and enumerated all resident adult household members.
CLIC
Eligible individuals providing written informed consent responded to a socio-demographic and sexual behaviour questionnaire and gave a finger prick sample collected as a dried blood spot, used for HIV incidence estimation. HIV counsellors also offered individuals point-of-care rapid HIV counselling and testing.
CLIC
Home-based survey rounds were repeated approximatively every six months. The list of resident household members was updated and exits (including deaths and out-migration from trial area) were documented.
CLIC
All trial participants identified as HIV-positive (through rapid HIV test or self-report) were referred to a local trial clinic set up by the trial and situated in the trial cluster in which they lived, located at less than 45 minutes walking distance.
In the trial clinics of the control clusters, HIV-positive adults were offered ART according to national guidelines. In the trial clinics of the intervention clusters, all HIV-positive adults were offered the opportunity to begin ART immediately regardless of CD4 count or clinical staging.
CLIC
The trial area was also served by three local governmental primary care clinics of the department of health providing HIV testing, care and treatment according to national guidelines only. HIV-positive participants of both arms could opt to receive HIV care in primary care clinics or transfer to a trial clinic. With the authorization of the ethical committee, we were able to link individual level data from the local governmental clinics with our trial data.

The trial was implemented in 22 clusters in 3 steps: 4 clusters opened in 2012, 6 clusters in 2013 and 12 clusters in 2014. All clusters were followed until mid-2016. Therefore, the number of survey rounds and follow-up time differ per cluster.
Two years ago, we presented the main trial results in Durban.
We did not observe a significant difference in HIV incidence between trial arms.
CLIC
However, did Population Viral Suppression improve during the course of the trial? Differentially by arm? According to trial interventions or contextual changes, independent of the trial?
The denominator used for computing Population Viral Suppression at cluster level, i.e. the local adult resident HIV-infected population, was changing over time due to in-migration, 16th birthday, HIV seroconversions, out-migrations and deaths.
CLIC
Population viral suppression corresponds to the proportion of residents adults living with HIV being in care, on ART and virally suppressed.
CLIC
It was computed at different time points: a pre-intervention estimate, considering the situation of individuals at the initial census of the population performed during the first round of each cluster, plus an estimate per day once the initial census of the population was completed.
At baseline, population viral suppression was similar between arms but slightly lower in intervention arm.
CLIC
It increased significantly in both arms between pre-intervention and January first 2016: +23 in intervention arm and +19 in control arm.
CLIC
That increase remained similar between arms, although the increase was slightly better in intervention arm, difference in differences being of +4.
CLIC
Therefore, at the end of the trial, population viral suppression was not significantly different between arms.
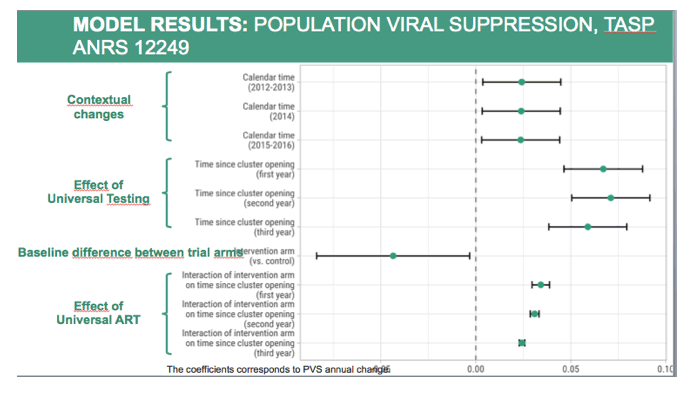
To disaggregate effects due to trial interventions implemented in both arms or intervention arm only and contextual changes, we used a mixed linear model to explore the relation between population viral suppression with calendar time, time since cluster opening, trial arm and interaction between arm and time since cluster opening, adjusting on sociodemographic changes at cluster level and including a random effect on cluster.

This graph represents the coefficients estimated by the model.
CLIC
Population viral suppression increase was mainly driven by time since cluster opening, measuring the impact of repeat home-based HIV testing and implementation of local trial clinics, both having been implemented in all clusters.
CLIC
As already seen, Population Viral Suppression at baseline was lower in the intervention arm.
CLIC
However, the increase was more important in the intervention arm, measuring the effect of initiating treatment regardless of CD4 count compared to national guidelines. The effect of Universal Treatment was smaller than the effect of Universal Testing, due to low level of linkage to care.
CLIC
Finally, they were also some effect due to contextual changes, measured by calendar time. In 2015, South Africa changed its treatment initiation guidelines, from 350 to 500 CD4 count.
Our analysis presents some limitations. In particular, population viral suppression is probably underestimated due to the fact that some trial participants receiving care in local governmental clinics were probably not successfully matched between governmental and trial database and therefore wrongly classified as not in care.
In addition, we did not capture HIV care received in private sector or outside the trial area.
Overall, 9.5% of the trial population had no observed HIV status and was excluded from the analysis. It could induce some overestimation as we can hypothesise that these individuals are less likely to receive care. However, we did perform some sensitivity analysis not presented here and results remained unchanged.

Although suboptimal, the UTT strategy implemented in TasP trial improved significantly population viral suppression over time.
As it was mainly due to universal testing rather than universal treatment, it did not induce difference between arms, explaining the null effect observed on cumulative incidence. Unfortunately, the trial was not powered to measure if incidence decreased over time within each arm, but to compare if overall incidence was different between arms.
CLIC
Changes in treatment initiation guidelines alone are not enough to significantly increase population viral suppression if no additional intervention is implemented to improve linkage to care in this rural setting.
|
| |
|
 |
 |
|
|