 |
 |
 |
| |
No Difference in MK-8591 and Doravirine
Pharmacokinetics After Co-administration
|
| |
| |
Reported by Jules Levin
IDWeek 2018; San Francisco, CA, USA; October 3-7, 2018.
Randolph P Matthews1; Deanne J Rudd1; Kerry L Fillgrove1;
Sabrina Fox-Bosetti1; Vanessa Levine1; Saijuan Zhang1;
Charles Tomek2; S Aubrey Stoch1; Marian Iwamoto1
1Merck & Co., Inc., Kenilworth, NJ, USA; 2Celerion, Lincoln, NE, USA
Active, not recruiting
MK-8591 With Doravirine and Lamivudine in Participants Infected With Human Immunodeficiency Virus Type 1 (MK-8591-011)
A Phase 2B, Randomized, Double-Blind, Active-Comparator-Controlled, Dose-Ranging Clinical Trial to Evaluate the Safety, Tolerability, Antiretroviral Activity, and Pharmacokinetics of MK-8591 Given in Combination With Doravirine (DOR) and Lamivudine (3TC) in HIV-1-Infected Treatment-Na´ve Adults
Sponsor:
Merck Sharp & Dohme Corp.
This study will evaluate the safety, tolerability, antiretroviral activity, and pharmacokinetics of 3 doses of MK-8591 in combination with doravirine (DOR) and lamivudine (3TC) administered to antiretroviral treatment-na´ve adult participants with human immunodeficiency virus type 1 (HIV-1) infection. 120 participants.
- Experimental: MK-8591 0.25 mg
Participants will be treated once daily (QD) with 0.25 mg MK-8591, 100 mg DOR, 300 mg 3TC, and placebo to MK- 1439A for a minimum of 24 weeks. Between week 24 through week 52, 3TC and placebo to MK-1439A may be discontinued. Around Week 60, participants may be switched to a selected open label dose of MK-8591 and DOR 100 mg QD and continue treatment until Week 120.
- Experimental: MK-8591 0.75 mg
Participants will be treated QD with 0.75 mg MK-8591, 100 mg DOR, 300 mg 3TC, and placebo to MK- 1439A for a minimum of 24 weeks. Between week 24 through week 52, 3TC and placebo to MK-1439A may be discontinued. Around Week 60, participants may be switched to a selected open label dose of MK-8591 and DOR 100 mg QD and will continue treatment until Week 120.
- Experimental: MK-8591 2.25 mg
Participants will be treated QD with 2.25 mg MK-8591, 100 mg DOR, 300 mg 3TC, and placebo to MK- 1439A for a minimum of 24 weeks. Between week 24 through week 52, 3TC and placebo to MK-1439A may be discontinued. Around Week 60, participants may be switched to a selected open label dose of MK-8591 and DOR 100 mg QD and will continue treatment until Week 120.
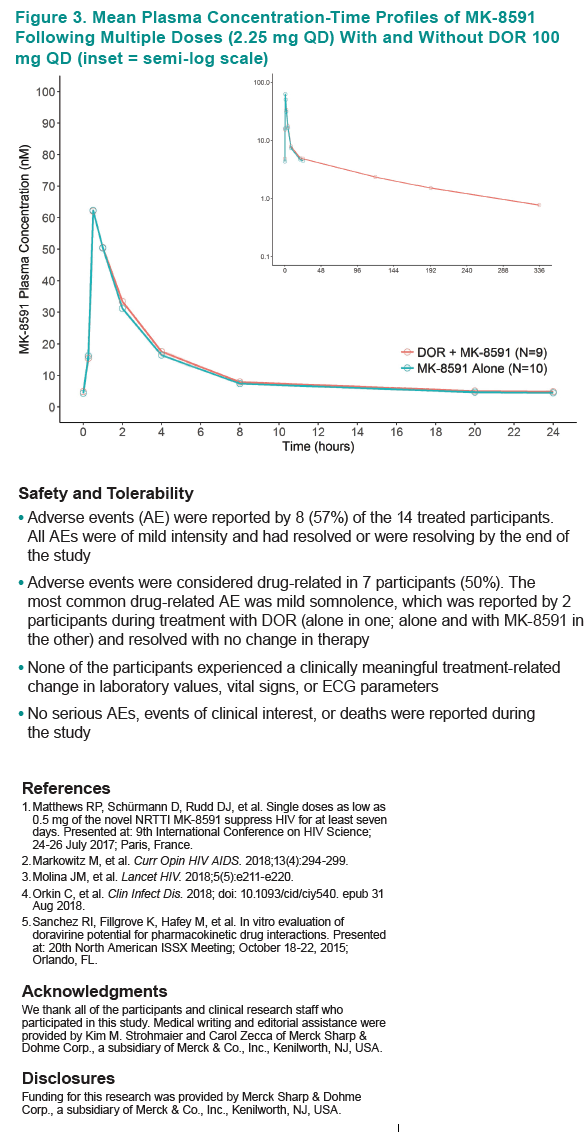
|
| |
|
 |
 |
|
|