 |
 |
 |
| |
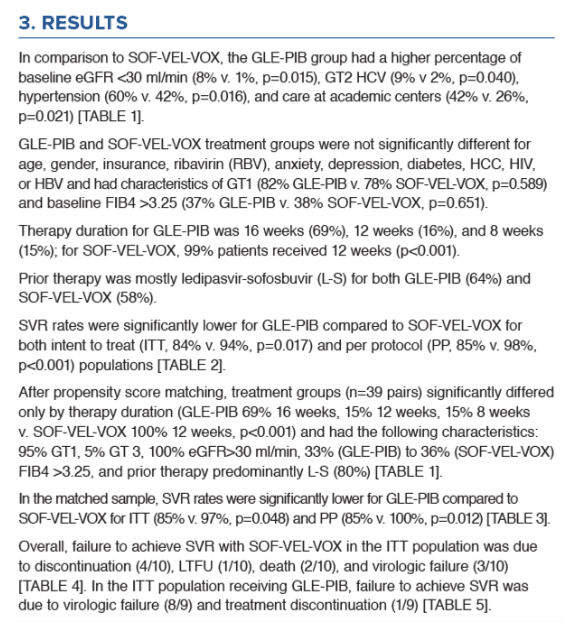
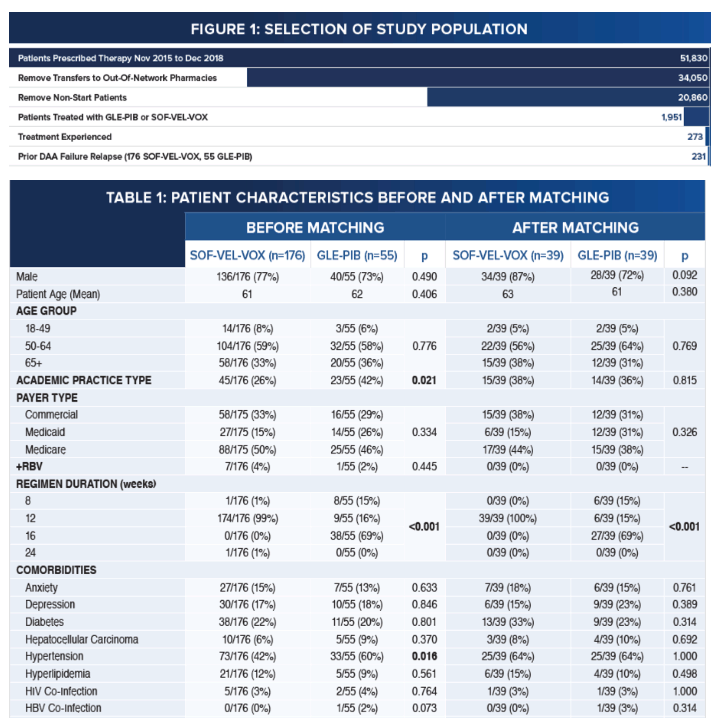
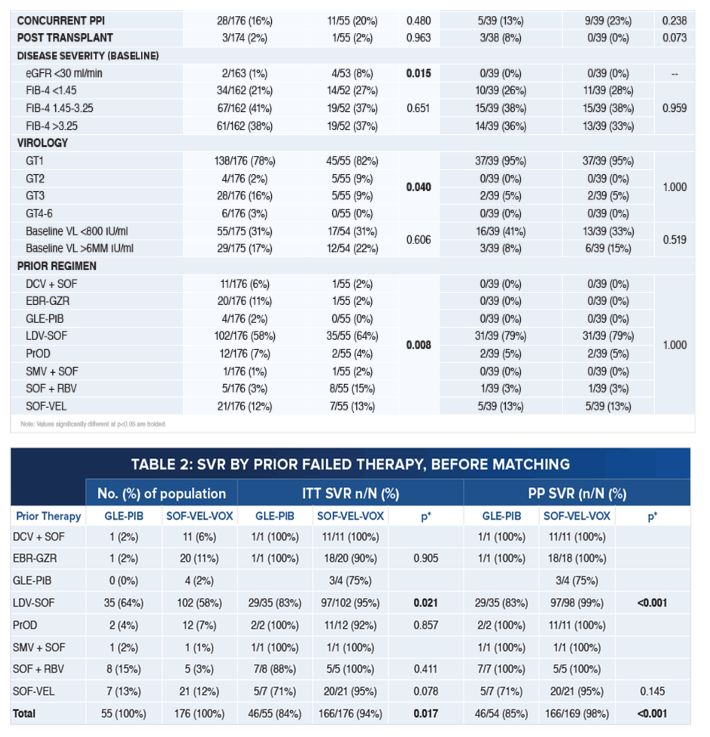
Pangenotypic Therapies Glecaprevir-Pibrentasvir (GLE-PIB) and Sofosbuvir-Velpatasvir-Voxilaprevir (SOF-VEL-VOX)
After Failure with Interferon (IFN)-Free Direct-Acting Antiviral (DAA) Treatment for Hepatitis C
|
| |
| |
AASLD 2019 Nov 8-12 Boston
Reported by Jules Levin
1Steven L Flamm, 2Naoky C Tsai, 3Bruce R Bacon, 4Michael P. Curry, 5Scott Milligan, 5Nicole Wick, 6Zobair M Younossi, 8Nezam H Afdhal
1Division of Hepatology, Northwestern University, Feinberg School of Medicine, Chicago, IL, USA, 2University of Hawaii, Honolulu, Hawaii, USA, 3Saint Louis University School of Medicine, St. Louis, Missouri, USA, 4Beth Israel Deaconess Medical Center, Boston, MA, USA, 5Trio Health Analytics, La Jolla, CA, USA,
6Center for Liver Diseases and Department of Medicine, Inova Fairfax Hospital, Falls Church, VA, USA
from EASL 2017:
MAGELLAN-1, PART 2: GLECAPREVIR/PIBRENTASVIR FOR 12 OR 16 WEEKS IN PATIENTS WITH CHRONIC HCV GENOTYPE 1 OR 4 AND PRIOR DIRECT-ACTING ANTIVIRAL TREATMENT FAILURE

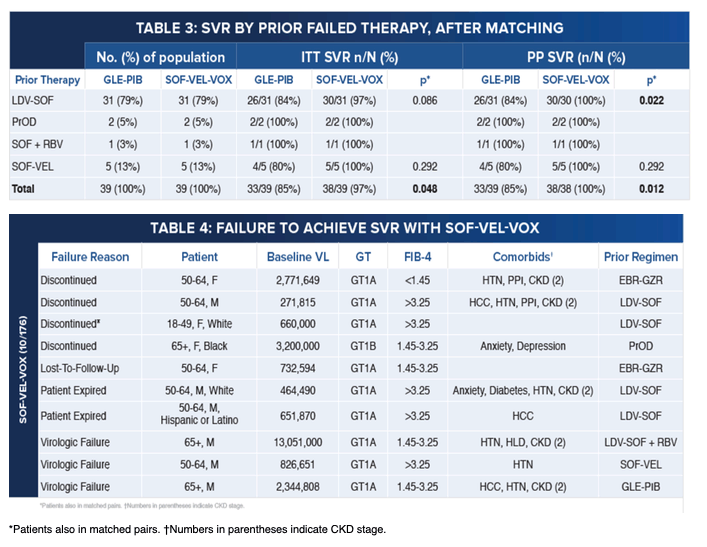
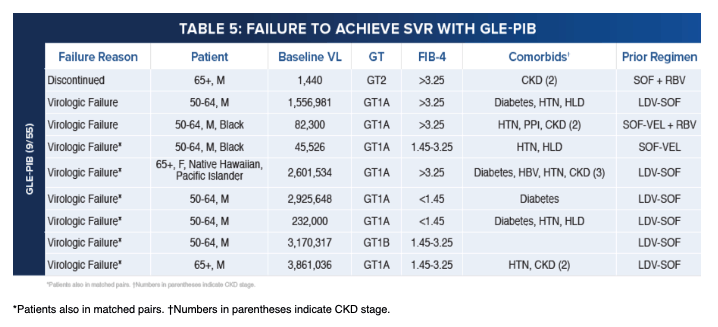
|
| |
|
 |
 |
|
|