 |
 |
 |
| |
GS-6207, A POTENT AND SELECTIVE FIRST-IN-CLASS LONG-ACTING HIV-1 CAPSID INHIBITOR
|
| |
| |
CROI: SAFETY AND PK OF SUBCUTANEOUS GS-6207, A NOVEL HIV-1 CAPSID INHIBITOR (03/08/19)
Reported by Jules Levin
CROI 2019 March 4-7 Seattle
Stephen R. Yant1, Andrew Mulato1, George Stepan1, Armando G. Villasenor1, Debi Jin1, Nicolas A. Margot1, Shekeba Ahmadyar1, Renee R. Ram1, John R. Somoza1, Eric Singer1, Melanie Wong1, Yili Xu1, John O. Link1, Tomas Cihlar1, Winston C. Tse1
1Gilead Sciences, Inc, Foster City, CA, USA
Program Abstract
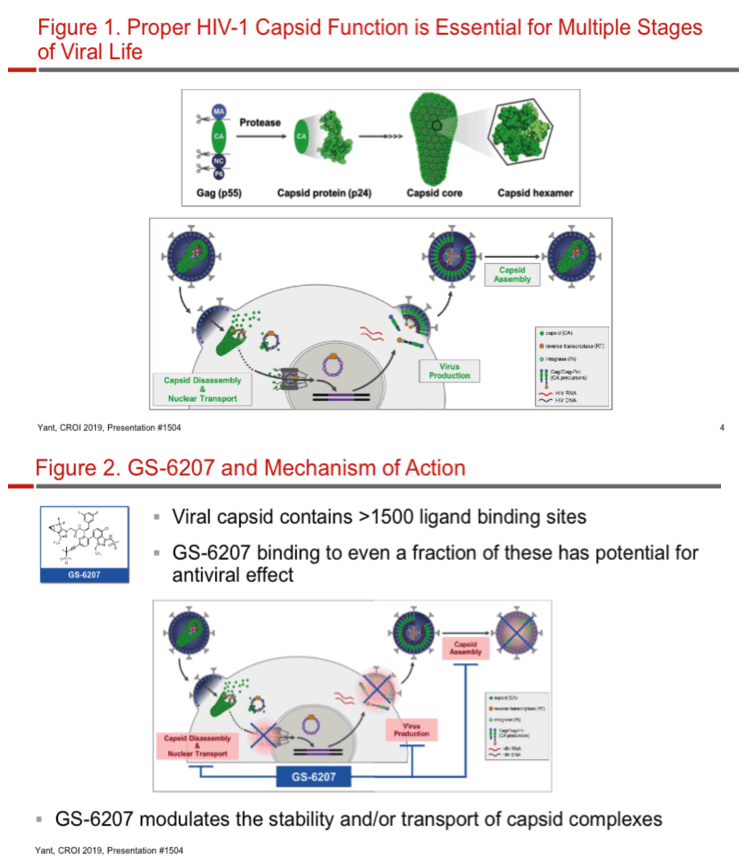
We describe the in vitro pharmacological profile of GS-6207, a first-in-class HIV capsid (CA) inhibitor optimized for long-acting antiretroviral (ARV) treatment administered monthly or less frequently.
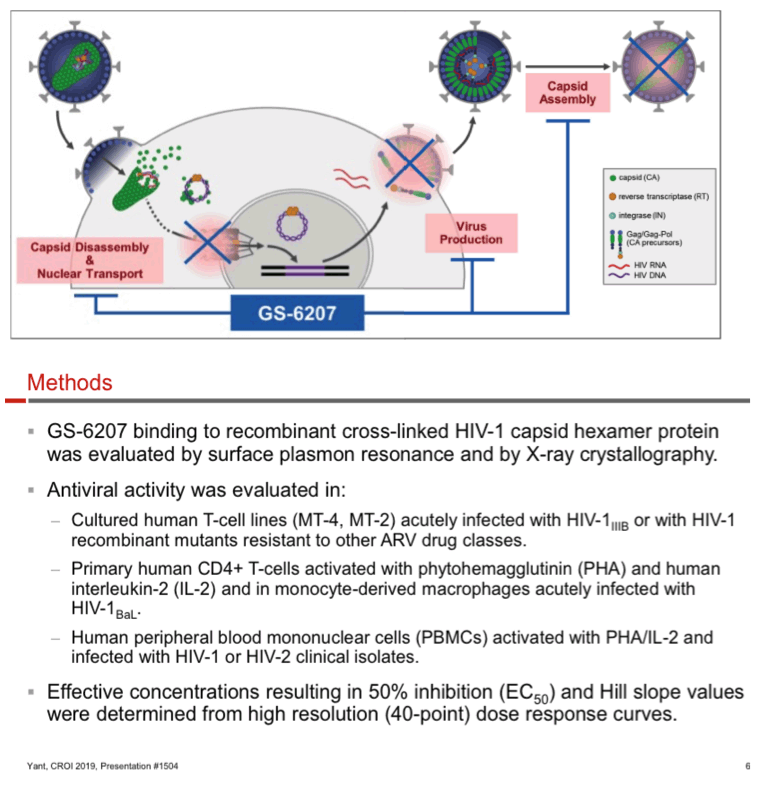
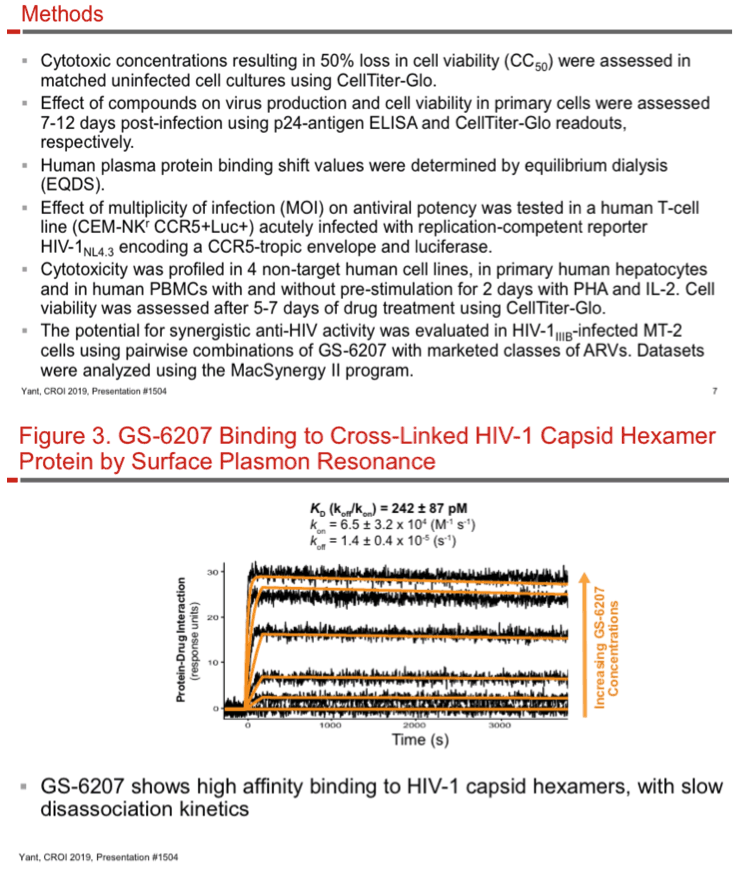
GS-6207 binding to HIV-1 CA hexamer was evaluated by surface plasmon resonance and x-ray crystallography. Antiviral potency and cytotoxicity were assessed in human T-cell lines and primary cells. HIV-1 and -2 laboratory strains and clinical isolates as well as HIV-1 recombinant mutants resistant to other ARV drug classes were used for antiviral profiling. Effect of the multiplicity of infection (MOI) on antiviral potency was tested using a reporter HIV-1. Cytotoxicity was profiled in 4 non-target human cell lines and primary hepatocytes. GS-6207 activity was evaluated in combination with marketed classes of ARVs.
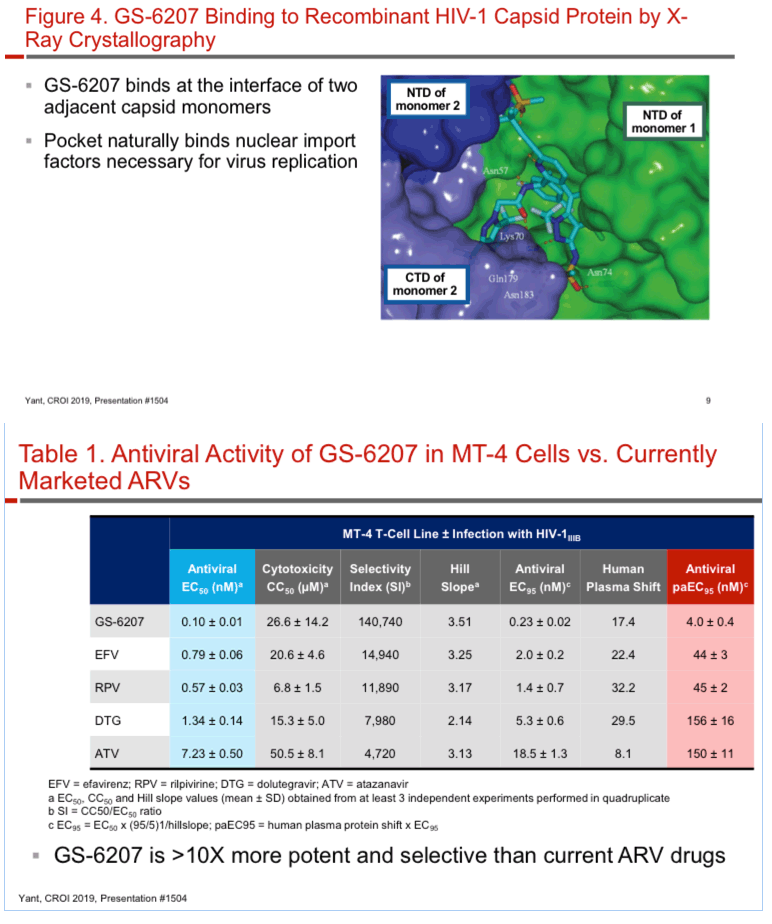
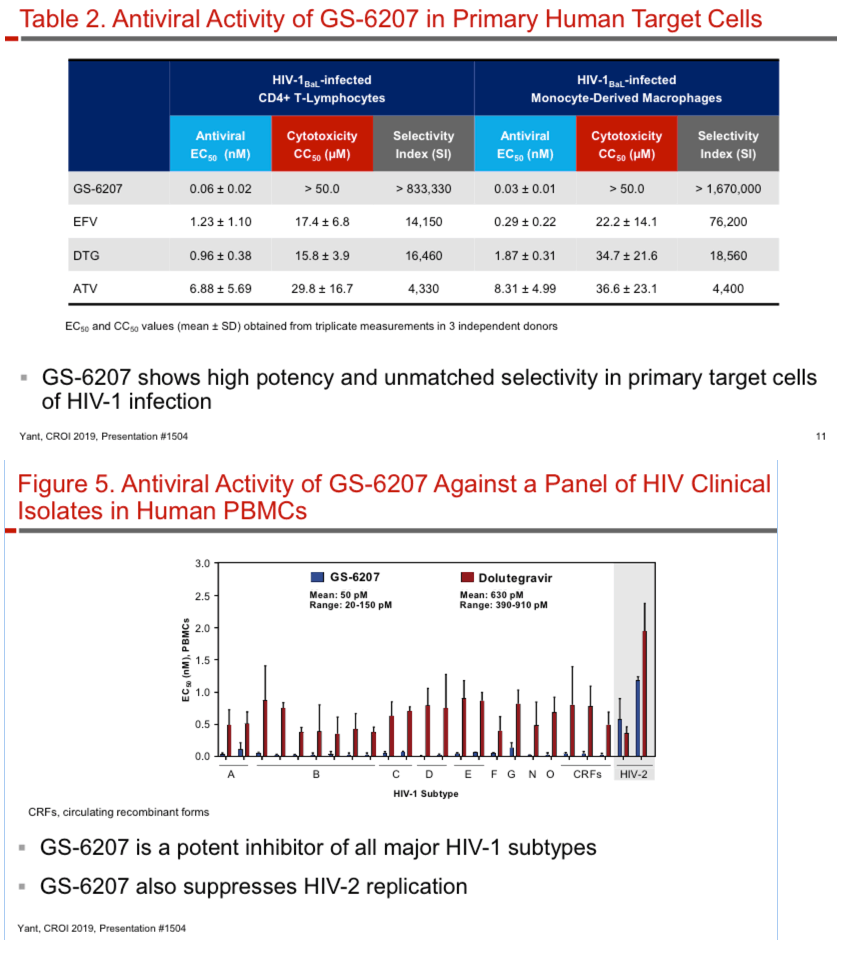
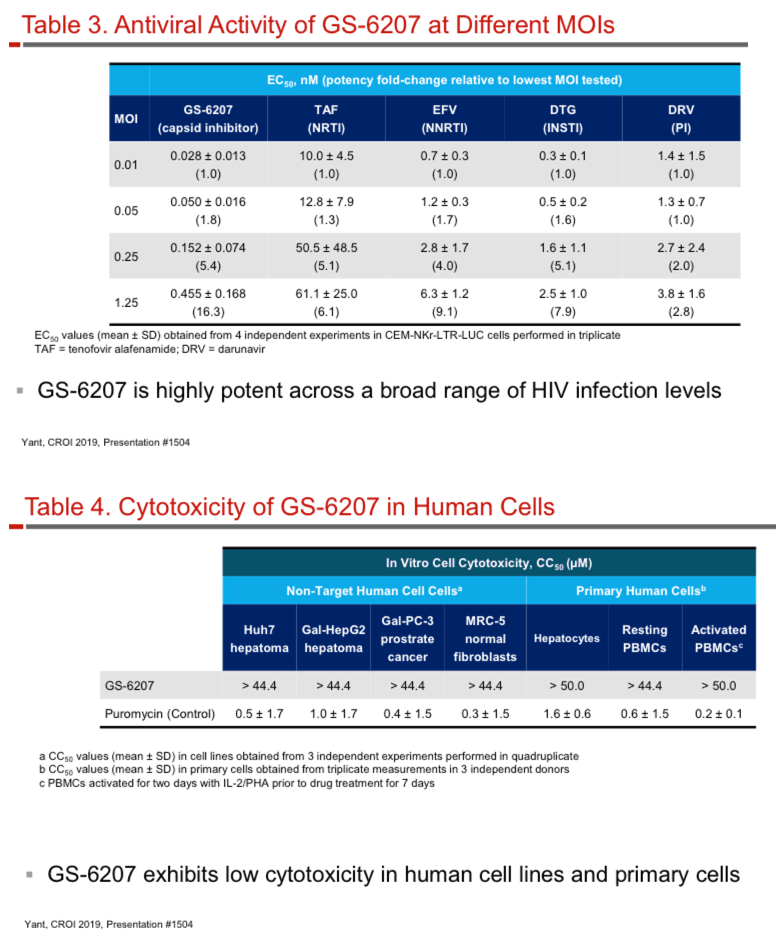
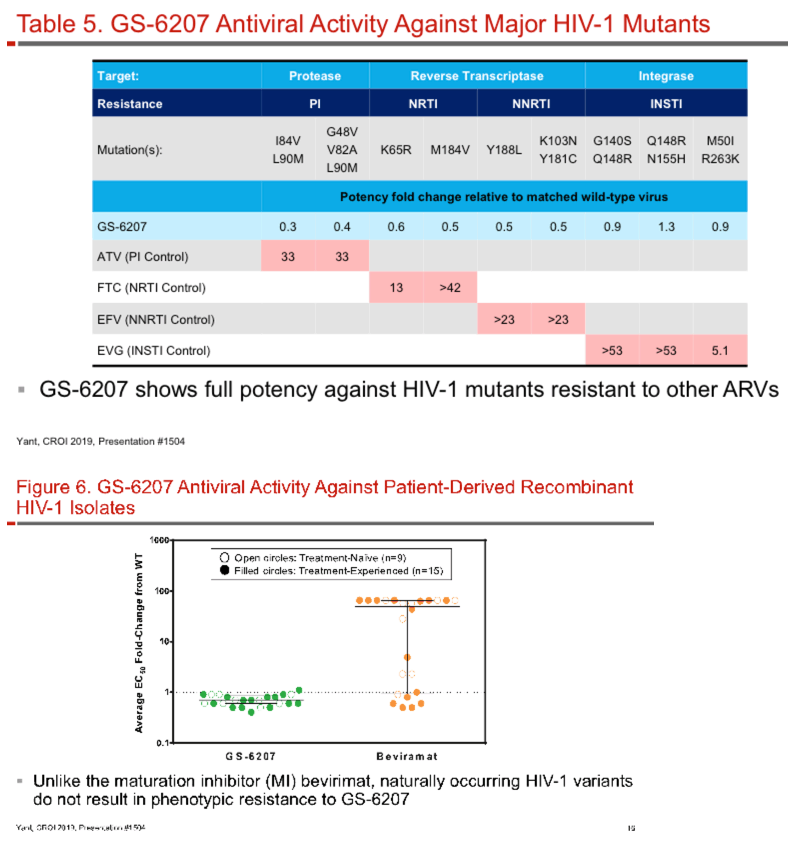
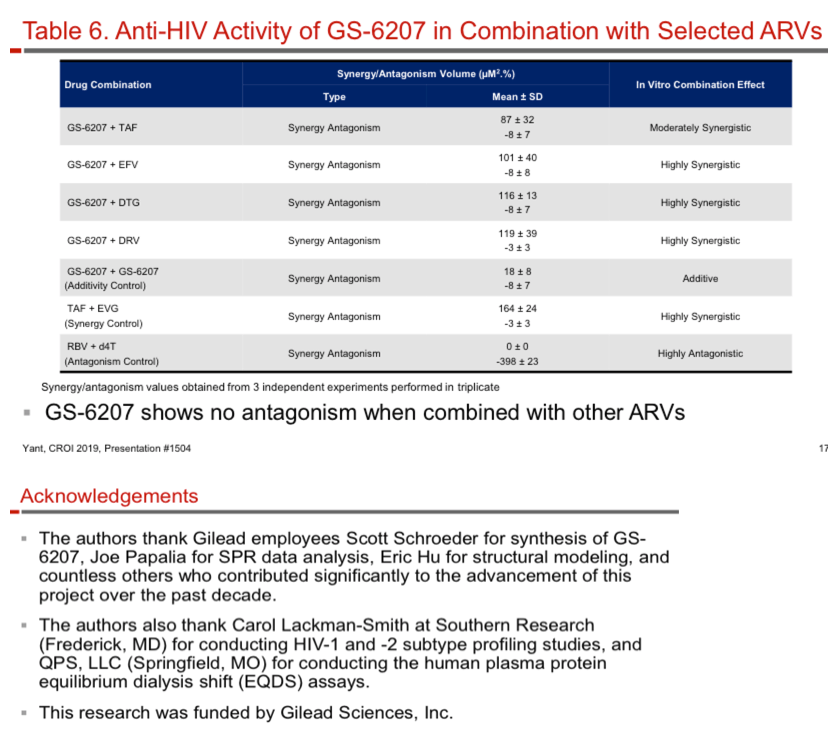
GS-6207 binds with high affinity to CA hexamers (KD = 0.2 nM) at the interface between two adjacent CA monomers. GS-6207 displays potent and selective antiviral activity in MT-4 cells (EC50 = 0.1 nM, CC50 = 27 µM) and exhibits a mean EC50 of 0.05 nM (0.02 - 0.16 nM) in human PBMCs against 23 HIV-1 clinical isolates spanning all major subtypes. The human serum protein-adjusted EC95 for GS-6207 (4 nM) is >10-fold lower than that of efavirenz (EFV), rilpivirine, dolutegravir (DTG) and atazanavir (ATV). In primary human CD4+ T-cells and macrophages, GS-6207 is >10-fold more potent and >22-fold more selective than EFV, DTG and ATV. GS-6207 also suppresses HIV-2 replication. As with other ARVs, GS-6207 antiviral activity decreases with increasing MOI but remains 5- to >100-fold more potent than 4 commonly used ARVs. GS-6207 exhibits low cytotoxicity in 4 human cell lines and primary hepatocytes (CC50 > 44 µM) and shows synergistic antiviral activity when combined pairwise with agents from each of 4 marketed ARV classes. Finally, GS-6207 retains full potency against a broad range of HIV-1 mutants resistant to other ARV classes, including those with naturally occurring Gag polymorphisms conferring resistance to maturation inhibitors.
GS-6207 is a novel HIV capsid inhibitor with picomolar potency and a unique resistance and pharmacokinetic (PK) profile that make it a suitable candidate for a low-dose long-acting subcutaneous administration to treat HIV-1 infection, including variants resistant to current ARV therapies. The safety and PK of GS-6207 is now being evaluated in healthy human subjects.


|
| |
|
 |
 |
|
|