 |
 |
 |
| |
Rebound Of HIV-1 In Cerebrospinal Fluid (CSF)
After Treatment Interruption (TI)
|
| |
| |
Reported by Jules Levin
CROI 2019 March 4-7 Seattle
Richard W. Price1, Maria Bednar2, Laura Kincer2, Rebecca Hoh1, Magnus Gisslen3, Sarah Joseph2, Serena Spudich4, Steven Deeks1, Ronald Swanstrom2
Background
HIV-1 persists in the body despite years of suppressive antiviral therapy. One well characterized viral reservoir is in resting CD4+ T cells. Under certain circumstances an independently replicating viral population can be detected in the CNS as indicated by differing viral sequences in blood and CSF. We examined rebound virus in the blood and CSF in 9 participants who chose to interrupt therapy.
Methods
Nine men interrupted therapy (TI), 6 in the context of early systemic failure (Price and Deeks J. Neurovirol. 2004 PMID: 14982739). Multiple blood and CSF samples were collected before and after TI over a period of up to 41 weeks, and clinical and laboratory data were recorded. Viral RNA was extracted from the plasma and CSF at each time point and viral populations examined using deep sequencing, with the sampling depth defined and sequencing errors corrected using Primer ID. Selected CSF samples were used to generate full length envgenes that were used to pseudotypevirus to test entry phenotype using Affinofilecells at high and low CD4 density to test for macrophage tropism.
Results
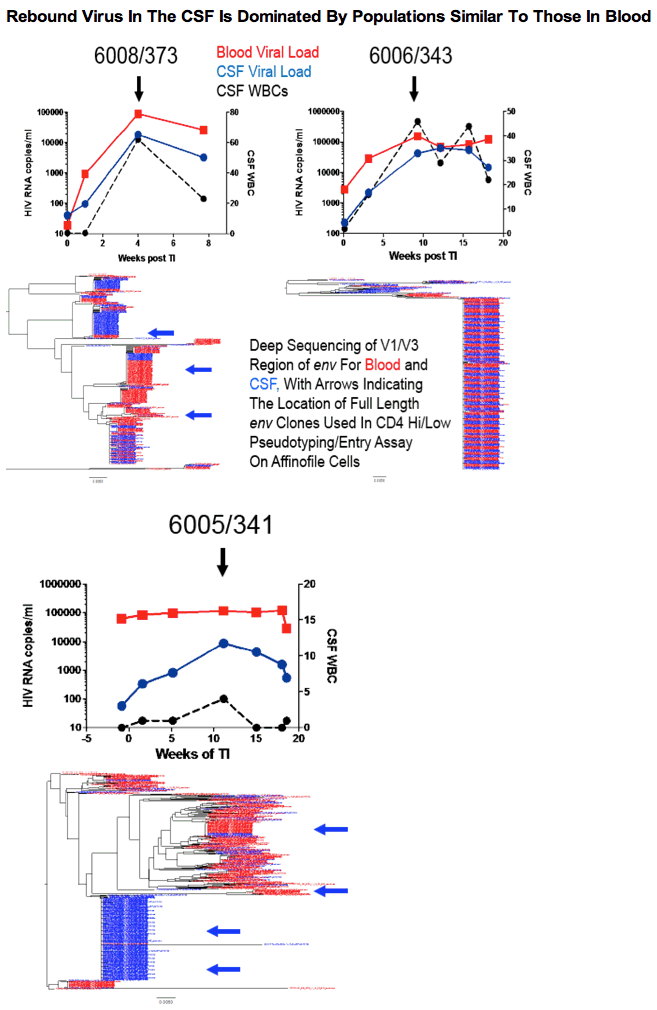
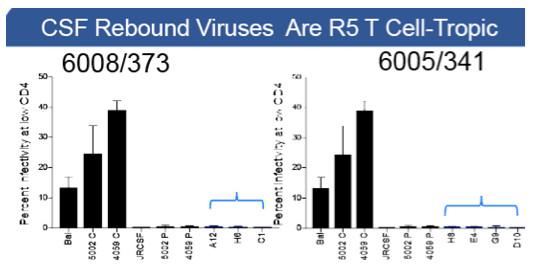
In 7/8 participants with clinical laboratory data available there was a significant increase in WBCs in the CSF, typically between 1-2 months after TI. The peak in WBCs in the CSF often corresponded with a peak in CSF viral load (VL), reaching viral RNA levels equivalent to those in the blood. At all analyzed time points for the 9 participants before and during the increase in VL in the CSF, the viral population in the CSF was well mixed with the viral population in the blood. Two frequently seen features of the CSF viral population were transient expansion of a single genotype (clonal amplification) of an R5 T cell-tropic virus, and differential penetration of one viral population present in the blood into the CSF relative to a second blood viral population. There was no evidence for a distinct viral population emerging from the CNS based on sampling of the CSF in these participants. Rebound virus in the blood had an entry phenotype consistent with being R5 T cell-tropic and not macrophage-tropic.
Conclusions
In this study of 9 participants we did not observe any evidence of a CNS-specific lineage in the CSF after TI in spite of extensively sampling the viral populations using deep sequencing. The large majority of the virus observed in the CSF appears to be the result of infected CD4+ T cells trafficking into the CNS from the blood rather than from 'release' from a CNS reservoir after TI. These results do not preclude a CNS reservoir but rather point to the difficulty of observing such a reservoir in the presence of actively circulating CD4+ T cells transporting virus from the blood into other compartments.

|
| |
|
 |
 |
|
|