| |
Increased Prevalence of Neurocognitive Impairment in Aging People Living With Human Immunodeficiency Virus: The ANRS EP58 HAND 55-70 Study; Commentary: Thinking About Getting Older With Human Immunodeficiency Virus "HIV Clinics Need To Expand Services"
|
| |
| |
Download the PDF here
Download the PDF here
"The development of combination antiretroviral therapy (cART) offers an extended life to people living with human immunodeficiency virus (HIV), fulfilling many of the dreams of therapeutic enthusiasts. That HIV, a stealthy but relentlessly lethal viral infection, can almost always be suppressed by a generally well-tolerated, single pill a day regimen is truly a spectacular scientific success. A cloud causing ongoing concern in this otherwise sunny story centers on the concern that the quality of this extended life might be inferior or even downright miserable for some people living with HIV, because of developing dementia. Living a life that becomes a burden to the patient or to caregivers would be a tragic twist to this saga.........Recognition that HIV in itself creates a more disabled population with special needs is a call to design care systems to meet the increased needs that this disability brings"
Increased Prevalence of Neurocognitive Impairment in Aging People Living With Human Immunodeficiency Virus: The ANRS EP58 HAND 55-70 Study.....35% had NCI neurocognitive impairment (24% ANI), 74% had increased risk for NCI, HIV associated with NCI; most NCI was ANI: asymptomatic neurocognitive impairment
Clinical Infectious Diseases 25 July 2019
NCI: 35% in HIV+ vs 24% in HIV-
ANI asymptomatic neurocognitively impaired - 19% in HIV- vs 24.5% in HIV+
MND mild neurocognitic disorders - 10.5% in HIV+ vs 4.7% in HIV-
Dementia: 0.3%(3) in HIV- vs 0.5% (1) in HIV+
see table 2 below
This was a cross-sectional study of PLHIV randomly matched by age (±4 years), gender, and education with 5 HIV-uninfected individuals from the CONSTANCES cohort. PLHIV were fluent in French and sequentially included during routine outpatient visits if aged 55-70 years, with HIV viral load <50 copies/mL, and lymphocyte T-CD4 level ≥200 cells/μL in the past 24 and 12 months, respectively. The primary outcome was NCI as defined by the Frascati criteria. Multivariate normative comparison (MNC) and -1.5 standard deviations in ≥2 neurocognitive domains were secondary outcomes of NCI. Results: Two hundred PLHIV were matched with 1000 controls. Median age was 62 years, and 85% were men. In PLHIV, the median T-CD4 lymphocyte level was 650 cells/μL, and median nadir T-CD4 lymphocyte level was 176 cells/μL. NCI was found in 71 (35.5%) PLHIV and in 242 (24.2%) controls (odds ratio [OR], 1.74; 95% confidence interval [CI], 1.25, 2.41). After adjusting for confounders, HIV remained significantly associated with NCI (OR, 1.50; 95% CI, 1.04, 2.16). Adjusted results were similar with NCI defined by MNC (ORMNC, 2.95; 95% CI, 1.13, 3.50) or -1.5 SD (OR-1.5, 2.24; 95% CI, 1.39, 3.62). Conclusions: In this matched study of aging individuals, HIV was significantly associated with an increased risk of NCI after adjusting for major confounders. Results were confirmed with more stringent NCI classifications.
-------------------------
Commentary: Thinking About Getting Older With Human Immunodeficiency Virus
"HIV Clinics Need To Expand Services"
"Recognition that HIV in itself creates a more disabled population with special needs is a call to design care systems to meet the increased needs that this disability brings"....."Finding a marked increase in neurocognitive impairments in aging PLHIV is a sobering task, and calls health-care providers to prepare additional support and therapy for the associated disability that this population suffers. However, there are also reassuring signs in this report. The majority of disabilities identified were asymptomatic or caused only a minor disability. Only 1 patient in 200 had dementia......The elephant in the room related to serious, age-related cognitive decline is Alzheimer disease (AD). While the prevalence of AD in the general population rises above age 70, it is logical that, if there were an acceleration of AD related to HIV, it should be emerging in this population as PLHIV approach age 70. That was not seen. While the clinical onset of AD is in older patients, biomarkers of AD are present in the brains of patients at least 15-20 years before the onset of dementia. The pattern of markers associated with AD has not been identified in PLHIV as a group, making it unlikely that AD is prematurely expressed in PLHIV [3]. Further, there are actually only a very few case reports of documented AD in any HIV patients [4]. These observations should lend hope that the doomsday scenarios around AD in HIV patients are unfounded."
"Recognition that HIV in itself creates a more disabled population with special needs is a call to design care systems to meet the increased needs that this disability brings....more isolated living status reflected in the demographics of this population, where more than a third live alone..... it is the responsibility of physicians to continue to advocate for our patients, and this report emphasizes significant and ongoing disabilities that we have yet to reverse and which are associated with HIV. Our HIV clinics will need to expand services to protect and support these patients appropriately. Meanwhile, prospective studies will be required to understand the details of interactions with HIV, its treatment, and associated conditions as patients enter the later years of their lives."
Clinical Infectious Diseases, 25 July 2019
David B. Clifford Department of Neurology, St. Louis, Missouri
The development of combination antiretroviral therapy (cART) offers an extended life to people living with human immunodeficiency virus (HIV), fulfilling many of the dreams of therapeutic enthusiasts. That HIV, a stealthy but relentlessly lethal viral infection, can almost always be suppressed by a generally well-tolerated, single pill a day regimen is truly a spectacular scientific success. A cloud causing ongoing concern in this otherwise sunny story centers on the concern that the quality of this extended life might be inferior or even downright miserable for some people living with HIV, because of developing dementia. Living a life that becomes a burden to the patient or to caregivers would be a tragic twist to this saga.
In this issue, Makinson and colleagues have provided a well-crafted analysis of the current status of aging people living with HIV (PLHIV) who have survived to have chronic, well-controlled HIV infections on current cART. The analysis provides hope, while raising realistic concerns that must be addressed in future studies. The manuscript reports the outcome of neurologic performance studies performed on 200 sequentially selected, HIV virally controlled PLHIV, compared with a matched (age, education, gender) community population of 1000. The study confirms that these PLHIV had worse neurocognitive performance than did controls. The primary analysis used the Frascati definition of impairment of at least 1 standard deviation (SD) in 2 domains, which, even when adjusted for confounders, gave an odds ratio of 1.5 (confidence interval 1.04-2.16) for neurocognitive impairment. More conservative methods, including increasing the SD to 1.5 to define impairment, or a more conservative multivariate normative comparison analysis, all supported the presence of HIV-associated impairments. The report has many strengths, including the use of the large, matched control group from a community study that provided contemporaneous norms; focusing the study on virally controlled patients without other seriously confounding illnesses; and sequentially selecting clinic patients to fill an age-balanced cohort representing ages 55-70 years. Confounders included in the adjusted analysis were cardiovascular disease, hypertension, diabetes, smoking, alcohol use, physical activity, depressive symptoms, and living alone. The fact that HIV remained associated with cognitive dysfunction in this older group is important.
Recognition that HIV in itself creates a more disabled population with special needs is a call to design care systems to meet the increased needs that this disability brings. This is particularly true given the more isolated living status reflected in the demographics of this population, where more than a third live alone. This isolation not only threatens support systems, but challenges the optimal ways for physicians to recognize, measure, and follow the real impacts of cognitive disabilities. In the setting of Alzheimer disease, an optimal means of understanding the true impact of advancing cognitive loss is through the clinical dementia rating (CDR) scale. This relies on an informant: generally someone living with the patient. Partners are sensitive to changes in behavior in everyday life and, with structured interviews, can alert and quantify changes remarkably well. With HIV patients often lacking such an observer, advancing disability may go unreported, and needed support will be delayed. This disadvantage is compounded due to the characteristics of the frontal predominant damage often seen in HIV brains, which may make patients less aware of their own deficits. Proactive care systems will be needed to identify and seek to ameliorate this impairment.
Having established that HIV populations experience more impairments for their age than controls, a very critical issue is whether the decline is accelerating. Aging comes with a predictable degree of progressive declines in performance on tests, and there is no expectation that HIV will be protective. However, if the persistent viral infection, drugs required to control it, or associated injuries accelerate the decline, aging would become exponentially difficult for HIV patients. Investigators have raised this specter in recent years, and caused considerable alarm for PLHIV. In this careful study examining a span of 15 years of aging, up to age 70, evidence was not found to suggest an acceleration of change by age. However, the authors were appropriately cautious not to claim that this is a reliable observation, noting that this study was underpowered to demonstrate such an association. Superficially, it would seem that this large a study would detect meaningful age-related changes. However, survival bias and the changing historical patterns of the epidemic make a cross-sectional analysis of these populations particularly treacherous. More severely impaired patients may have died at earlier ages, leaving increasingly mildly affected patients represented in older cohorts. Therapy has changed rapidly through the years, and survivors with different durations of infection will likely have had very different therapeutic histories. Classes of antiretroviral drugs keep changing, as has timing of the initiation of therapy. The fact that the duration of infection is not tightly linked to age further complicates the analysis. These factors will require longitudinal comparisons to ascertain that HIV and its therapies are not accelerating the development of impairment during controlled infections. Longitudinal observations currently are not uniformly coherent with regard to the progression of disability. Shorter, well-matched studies, such as those reported by Cole et al [1], have not found acceleration. Imaging analyses similarly did not find accelerating atrophy in the brains of PLHIV who were receiving effective therapy [2].
The elephant in the room related to serious, age-related cognitive decline is Alzheimer disease (AD). While the prevalence of AD in the general population rises above age 70, it is logical that, if there were an acceleration of AD related to HIV, it should be emerging in this population as PLHIV approach age 70. That was not seen. While the clinical onset of AD is in older patients, biomarkers of AD are present in the brains of patients at least 15-20 years before the onset of dementia. The pattern of markers associated with AD has not been identified in PLHIV as a group, making it unlikely that AD is prematurely expressed in PLHIV [3]. Further, there are actually only a very few case reports of documented AD in any HIV patients [4]. These observations should lend hope that the doomsday scenarios around AD in HIV patients are unfounded. Fortunately, tools to predict cognitive impairments with AD are improving rapidly [5, 6]. Amyloid and tau positron emission tomography imaging allow noninvasive measures of the key markers associated with AD. More encouraging is the prospect of a serum amyloid measurement sensitive enough to screen for preclinical AD [7]. As more cases of AD in HIV patients are seen in coming years, a careful analysis of the characteristic evolution of AD markers in the setting of HIV-Associated Neurocognitive Disorder (HAND) will be required. However, there is good reason to expect that the biomarker-based, positive identification of AD will be possible. Furthermore, the typical clinical course of AD is recognizable. AD is reliably progressive once it causes disability, whereas in a substantial majority of HAND patients, progression was not evident over some years of longitudinal follow-up [8]. While it will be important to further characterize the interaction of HAND and AD as our population enters the ages in which AD is prevalent, it seems likely that the differentiation will not be too difficult. What remains to be accomplished is the discovery of a disease-modifying therapy that could be provided early in the course so that the tragic disability of AD is averted.
Finding a marked increase in neurocognitive impairments in aging PLHIV is a sobering task, and calls health-care providers to prepare additional support and therapy for the associated disability that this population suffers. However, there are also reassuring signs in this report. The majority of disabilities identified were asymptomatic or caused only a minor disability. Only 1 patient in 200 had dementia. Further, the mix of these problems did not worsen with successively older cohorts, up to age 70. While the study of survivors is, by nature, a selected population, this older population of PLHIV might be predicted to have maximal deficits. They reflect long-abandoned care patterns, as a majority experienced periods of advanced disease with a median CD4 nadir below 200, and suffered acquired immunodeficiency syndrome-defining complications. In future aging populations, PLHIV who are now receiving more virologically potent therapies with fewer side effects, starting treatment immediately on a diagnosis of HIV, and in general starting treatment long before the immune system has collapsed, could see fewer impairments.
However, it is the responsibility of physicians to continue to advocate for our patients, and this report emphasizes significant and ongoing disabilities that we have yet to reverse and which are associated with HIV. Our HIV clinics will need to expand services to protect and support these patients appropriately. Meanwhile, prospective studies will be required to understand the details of interactions with HIV, its treatment, and associated conditions as patients enter the later years of their lives.
------------------
Increased Prevalence of Neurocognitive Impairment in Aging People Living With Human Immunodeficiency Virus: The ANRS EP58 HAND 55-70 Study.....we found a 74% increased risk of NCI in an aging population of PLHIV.....35% had NCI neurocognitive impairment (24% of these were asymptomatic, 74% had increased risk for NCI, HIV associated with NCI
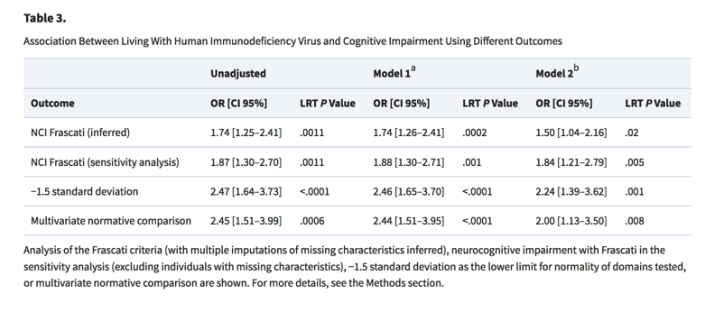
- We classified NCI in 71 PLHIV (35.5%) and in 242 controls (24.2%) (OR, 1.74; 95% CI, 1.25-2.41).
- Adjusting for the matching variables only in model 1, HIV was associated with NCI (OR, 1.74; 95% CI, 1.26-2.41). Further adjusting for confounders, that is, physical activity, diabetes, hypertension, cardiovascular disease, smoking status, CES-D ≥ 17, alcohol consumption, and living alone, HIV remained significantly associated with NCI (OR, 1.5; 95% CI, 1.04-2.16)
- Using the -1.5 SD from the norm cutoff classification, these numbers were 40 (20.0%) and 93 (9.3%) (unadjusted OR [OR-1.5], 2.47; 95% CI, 1.64-3.73). Using the MNC criteria, 27 (14.6%) PLHIV and 60 (6.6%) controls were impaired (unadjusted OR for MNC [ORMNC], 2.45; 95% CI, 1.51-3.99). In both multivariable analyses, HIV remained associated with cognitive impairment whether defined by -1.5 SD (OR-1.5, 2.24; 95% CI, 1.39-3.62) or MNC (ORMNC, 2.00; 95% CI, 1.13-3.50; Table 3).
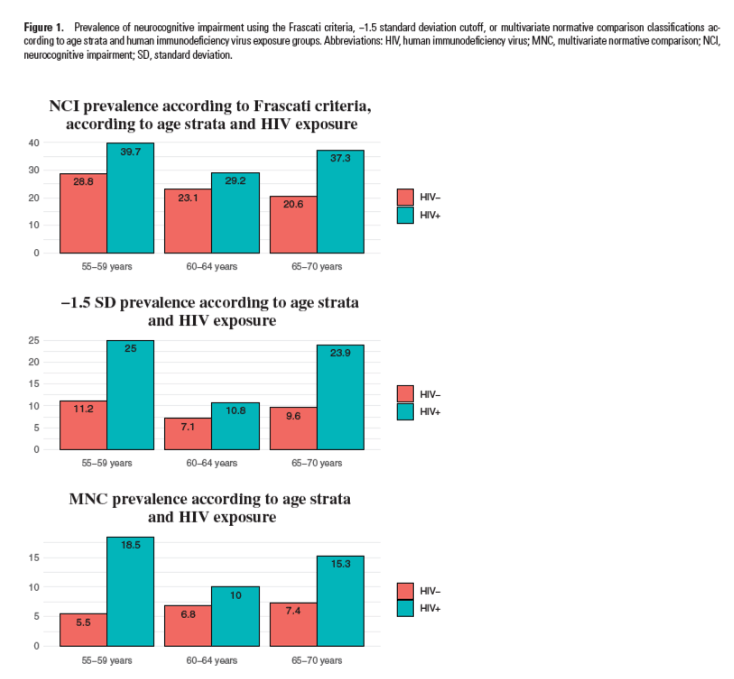
Based on the z score, prevalence of NCI using the Frascati criteria, -1.5 SD
classification, and MCI classifications according to age strata and HIV exposure groups are shown in Figure 1. No interaction between age and HIV status was objectified with any neurocognitive outcome (Table 4).
Clinical Infectious Diseases 25 July 2019
"we found a 74% increased risk of NCI in an aging population of PLHIV. This association persisted after adjusting for comorbid and social confounding factors and considering more stringent outcome measures......Using identical neurocognitive assessment, we found that the prevalence of NCI [18] was significantly increased in PLHIV aged >55 years compared to age, gender, and education matched controls and that HIV was independently associated with NCI after adjusting for important confounders. Using a more conservative threshold for definition of an abnormal domain of less than -1.5 SD from the norm and MNC, HIV was still associated with NCI.
"We classified NCI in 71 PLHIV (35.5%) and in 242 controls (24.2%) (OR, 1.74; 95% CI, 1.25-2.41).
use of the 4-question IADL instead of the 8-question IADL or a large panel of functional tests might have underestimated functional impairment. Also, survival bias, which is inherent to our cross-sectional design, may have selected increasingly fit individuals and limited the number of individuals with MND......The increased prevalence of cardiovascular risk factors, cannabis consumption, hypertension, and chronic kidney disease is in line with previous studies [16, 33], as well as the increased rate in depressive symptomatology [34]. Multimorbidity, to which aging PLHIV are more prone than their counterparts not living with HIV, has been linked in the general population to mental health issues, including depression [35]. The differences in social behavior are important, particularly in physical activity and living conditions, as well as the rates of depression in our study. These factors may explain, at least in part, the increased prevalence of NCI in PLHIV, but HIV remained significantly associated with NCI after adjusting for them."
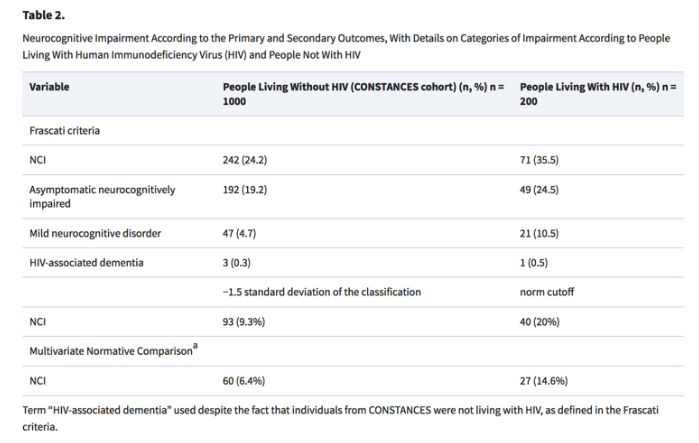
Distributions of NCI according to the Frascati criteria in both populations are shown in Table 2. Most impaired individuals were asymptomatic neurocognitively impaired (ANI). Only 3 individuals (0.30%) from the CONSTANCES cohort and 1 PLHIV (0.05%) were classified as having HIV-associated dementia. Neurocognitive tests significantly differed between groups in the TMT-A, TMT-B, and WAIS-IV (Supplementary Figure 1).
Adjusting for the matching variables only in model 1, HIV was associated with NCI (OR, 1.74; 95% CI, 1.26-2.41). Further adjusting for confounders, that is, physical activity, diabetes, hypertension, cardiovascular disease, smoking status, CES-D ≥ 17, alcohol consumption, and living alone, HIV remained significantly associated with NCI (OR, 1.5; 95% CI, 1.04-2.16). A sensitivity analysis that excluded individuals with 1 or more missing variables from the models did not substantially change our findings as HIV remained associated with NCI in the final model (OR, 1.84; 95% CI, 1.24-2.70; Table 3)."
NCI: 35% in HIV+ vs 24% in HIV-
ANI asymptomatic neurocognitively impaired - 19% in HIV- vs 24.5% in HIV+
MND mild neurocognitic disorders - 10.5% in HIV+ vs 4.7% in HIV-
Dementia: 0.3%(3) in HIV- vs 0.5% (1) in HIV+
Adjusting for the matching variables only in model 1, HIV was associated with NCI (OR, 1.74; 95% CI, 1.26-2.41). Further adjusting for confounders, that is, physical activity, diabetes, hypertension, cardiovascular disease, smoking status, CES-D ≥ 17, alcohol consumption, and living alone, HIV remained significantly associated with NCI (OR, 1.5; 95% CI, 1.04-2.16). A sensitivity analysis that excluded individuals with 1 or more missing variables from the models did not substantially change our findings as HIV remained associated with NCI in the final model (OR, 1.84; 95% CI, 1.24-2.70; Table 3).
Using the -1.5 SD from the norm cutoff classification, these numbers were 40 (20.0%) and 93 (9.3%) (unadjusted OR [OR-1.5], 2.47; 95% CI, 1.64-3.73). Using the MNC criteria, 27 (14.6%) PLHIV and 60 (6.6%) controls were impaired (unadjusted OR for MNC [ORMNC], 2.45; 95% CI, 1.51-3.99). In both multivariable analyses, HIV remained associated with cognitive impairment whether defined by -1.5 SD (OR-1.5, 2.24; 95% CI, 1.39-3.62) or MNC (ORMNC, 2.00; 95% CI, 1.13-3.50; Table 3).
Based on the z score, prevalence of NCI using the Frascati criteria, -1.5 SD classification, and MCI classifications according to age strata and HIV exposure groups are shown in Figure 1. No interaction between age and HIV status was objectified with any neurocognitive outcome (Table 4).
Abstract
Background
There are limited data on the comparative prevalence of neurocognitive impairment (NCI) in aging people living with human immunodeficiency virus (PLHIV) and people not living with HIV.
Methods
This was a cross-sectional study of PLHIV randomly matched by age (±4 years), gender, and education with 5 HIV-uninfected individuals from the CONSTANCES cohort. PLHIV were fluent in French and sequentially included during routine outpatient visits if aged 55-70 years, with HIV viral load <50 copies/mL, and lymphocyte T-CD4 level ≥200 cells/μL in the past 24 and 12 months, respectively. The primary outcome was NCI as defined by the Frascati criteria. Multivariate normative comparison (MNC) and -1.5 standard deviations in ≥2 neurocognitive domains were secondary outcomes of NCI.
Results
Two hundred PLHIV were matched with 1000 controls. Median age was 62 years, and 85% were men. In PLHIV, the median T-CD4 lymphocyte level was 650 cells/μL, and median nadir T-CD4 lymphocyte level was 176 cells/μL. NCI was found in 71 (35.5%) PLHIV and in 242 (24.2%) controls (odds ratio [OR], 1.74; 95% confidence interval [CI], 1.25, 2.41). After adjusting for confounders, HIV remained significantly associated with NCI (OR, 1.50; 95% CI, 1.04, 2.16). Adjusted results were similar with NCI defined by MNC (ORMNC, 2.95; 95% CI, 1.13, 3.50) or -1.5 SD (OR-1.5, 2.24; 95% CI, 1.39, 3.62).
Conclusions
In this matched study of aging individuals, HIV was significantly associated with an increased risk of NCI after adjusting for major confounders. Results were confirmed with more stringent NCI classifications.
INTRODUCTION
Since the advent of combined antiretroviral therapy (cART), the life expectancy for people living with human immunodeficiency virus (PLHIV) has substantially increased in resource-rich settings [1, 2]. Causes of morbidity and mortality have shifted from AIDS to non-AIDS-defining diseases [3, 4], and studies have found high proportions of neurocognitive impairment (NCI) in PLHIV, ranging from 19% to 67% [5-10]. NCI prevalence has varied widely in studies due to differing demographic characteristics and comorbidity burdens of study individuals, as well as the neurocognitive tests and classifications that were used. In PLHIV taking cART with controlled HIV viremia, the prevalence of NCI has been lower, albeit still substantial, ranging from 19% to 30% [5-7]. The incidence of NCI is expected to rise in PLHIV as the population ages [11]. There are, however, limited data on NCI prevalence or incidence in older PLHIV [5-10].
NCI prevalence may be higher in PLHIV compared to the general population. This could solely reflect the increased prevalence of NCI-associated risk factors in this population [12-17], such as cardiovascular disease or depression. Additionally, HIV infection may also be an additional risk factor and potentially the primary contributor of NCI in PLHIV [18], as studies have shown an association between NCI and HIV-related factors such as detectable viral load [6], current CD4 levels [19], nadir CD4 levels [6, 20], and history of AIDS [21]. However, clinical studies have found contradictory results. In the CIPHER study, there was no significant difference in severity or prevalence of NCI in 248 PLHIV and 45 men who have sex with men (MSM) not living with HIV [22]. In the MACS cohort, there was no association between HIV and NCI in 428 PLHIV and 207 MSM not living with HIV after adjusting for education, depression, and race [23]. The COBRA cohort showed, however, baseline poorer global cognitive performances in 134 virologically controlled PLHIV and 79 demographically similar controls not living with HIV, but the association with HIV was not addressed [24].
Further studies to evaluate the prevalence of NCI in aging PLHIV are thus needed, as well as studies to assess the association between HIV and NCI. In our study, we compared the prevalence of NCI in PLHIV aged 55 to 70 years with controlled viral load with an age, gender, and education matched control population of people not living with HIV, adjusting for a wide variety of confounding factors including behavioral and social factors
DISCUSSION
Using identical neurocognitive assessment, we found that the prevalence of NCI [18] was significantly increased in PLHIV aged >55 years compared to age, gender, and education matched controls and that HIV was independently associated with NCI after adjusting for important confounders. Using a more conservative threshold for definition of an abnormal domain of less than -1.5 SD from the norm and MNC, HIV was still associated with NCI.
In our study, prevalence of NCI according to the Frascati criteria (35.5%) was in line with the prevalence in studies of individuals with controlled HIV viremia of between 19% and 30% [5-7]. The slightly higher prevalence in our study could be due to the skewed higher median age, as most previous studies were performed in younger populations. The fact that the majority of NCI cases classified as ANI followed by MND is in line with previous studies of HAND in PLHIV [6, 9]. Despite being valid, use of the 4-question IADL instead of the 8-question IADL or a large panel of functional tests might have underestimated functional impairment. Also, survival bias, which is inherent to our cross-sectional design, may have selected increasingly fit individuals and limited the number of individuals with MND. Moreover, our results of increased prevalence of NCI in PLHIV seem robust, as the adjusted ORs of HIV were still significantly associated with NCI in HAND sensitivity analyses (excluding individuals with missing characteristics) and using the more stringent definitions of less than -1.5 SD from the norm in 2 neurocognitive domains and MNC.
The increased prevalence of cardiovascular risk factors, cannabis consumption, hypertension, and chronic kidney disease is in line with previous studies [16, 33], as well as the increased rate in depressive symptomatology [34]. Multimorbidity, to which aging PLHIV are more prone than their counterparts not living with HIV, has been linked in the general population to mental health issues, including depression [35]. The differences in social behavior are important, particularly in physical activity and living conditions, as well as the rates of depression in our study. These factors may explain, at least in part, the increased prevalence of NCI in PLHIV, but HIV remained significantly associated with NCI after adjusting for them.
Interestingly, it is in the COBRA cohort in which PLHIV and the control group (median age 56 years) had similar demographic and lifestyle characteristics that a difference in NCI prevalence was revealed [24]. In the MACS cohort (median age 48 years) [23], HIV status was not significantly associated with poorer cognitive test performances. In this cohort, the lower median ages, rates of AIDS history (11%), high median T-CD4 lymphocyte nadir (295 cells/μL), and prevalence of cardiovascular factors, depressive symptomatology, and recreational drug use may have contributed to the lack of association.
Aging is an important factor in the cognitive decline in PLHIV as well as in the general population [36, 37]. There was no interaction between age and HIV on associations with NCI, despite an age span of 15 years. However, the cross-sectional nature of the analysis and a lack of power to address this issue hinder any firm conclusion on a potential interaction, which would benefit from further studies. Also, survival bias limits interaction analysis of age with HIV. In the COBRA study [24], there was no evidence of accelerated brain pathology in PLHIV who underwent magnetic resonance imaging (MRI) or of neurocognitive performance after a median follow-up of 2 years, but the MACS cohort showed a greater-than-expected effect of aging on episodic memory and motor function in individuals with AIDS [38].
The association between HIV and NCI is probably related to multifactorial etiologies. HIV-specific patterns of brain abnormality on MRI have recently been identified and could be the result of chronic brain immune activation despite controlled HIV viremia [39-41]. ANI was associated with specific frontal white matter atrophy, while MND was characterized by more widespread subcortical atrophy [40]. In another study, long-standing virologically controlled PLHIV were found to have widespread abnormalities in white matter microstructure, with abnormalities correlating with cognitive function and systemic immune activation [41]. In our study, the high rates of past immunosuppression may therefore have significantly impacted neurocognitive outcomes, as previously published [21].
Our study has several limits. Individuals recruited in the CONSTANCES cohort may have a health effect bias, as using volunteers may introduce selection bias, even in studies that use random recruitment from an appropriate sampling base [42]. However, matching on the education level in our study reduced the risk of this bias. Also, there were more missing variables in individuals with NCI in the CONSTANCES controls than in PLHIV, which could have overestimated NCI prevalence in PLHIV. However, the primary analysis, with missing variables imputed to include all individuals, limited the impact of this bias. Finally, despite all the matching and extensive adjustments, there might be remaining residual unaccounted confounders.
In conclusion, in this carefully age, gender, and education matched study, with a control group selected from a national cohort and using identical methods of neurocognitive assessment, we found a 74% increased risk of NCI in an aging population of PLHIV. This association persisted after adjusting for comorbid and social confounding factors and considering more stringent outcome measures.
|
|
| |
| |
|
|
|