 |
 |
 |
| |
Pregnancy outcomes following raltegravir exposure
|
| |
| |
No Link Between Raltegravir and Adverse Birth Outcomes in 2550-Woman Analysis
IDWeek, October 2-6, 2019, Washington, DC
Mark Mascolini
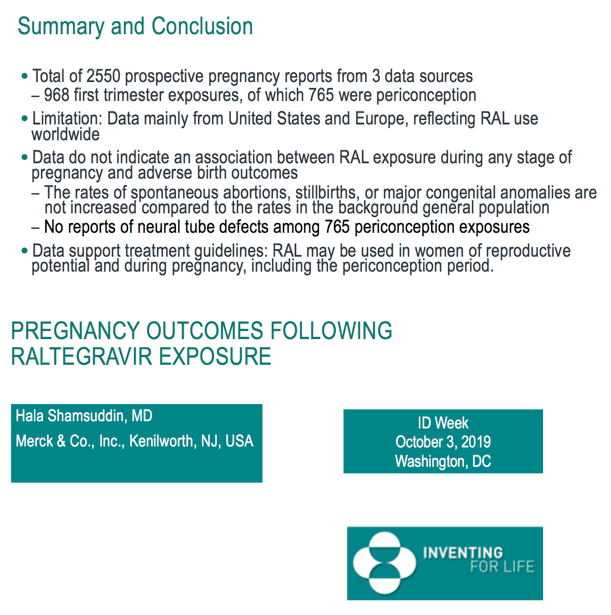
Analysis of 2550 prospective pregnancy reports found no association between raltegravir use and adverse birth outcomes [1]. Rates of spontaneous abortion, stillbirth, and major congenital anomalies matched those seen in the background population. Most of the reports came from the United States and Western Europe.
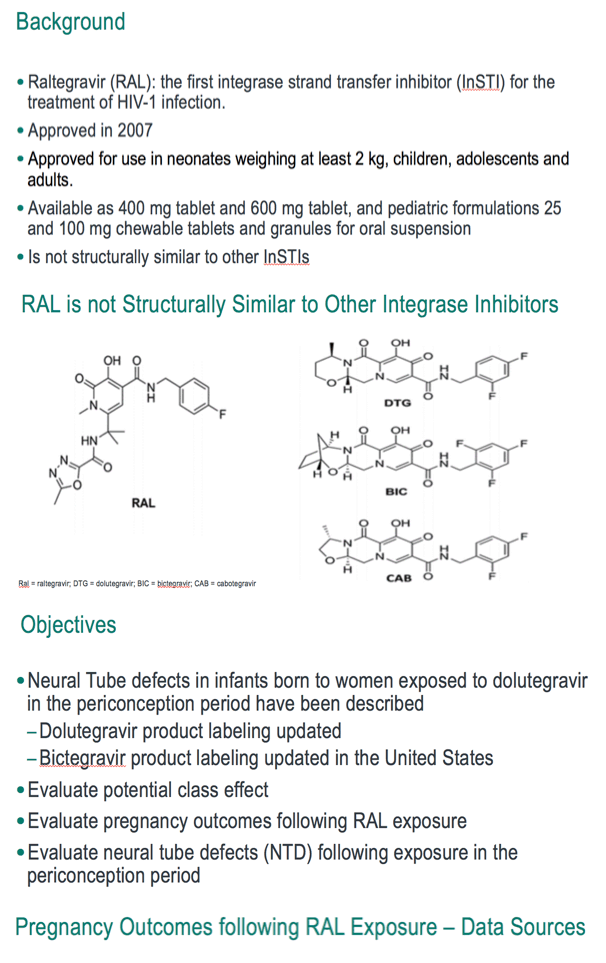
Not structurally similar to other HIV integrase inhibitors, raltegravir has been licensed since 2007 and is approved for use in neonates weighing at least 2 kg, children, adolescents, and adults. Neural tube defects have been described in infants of women taking dolutegravir in the periconception period. The purpose of this analysis was to assess all pregnancy outcomes among women taking raltegravir, especially potential neural tube defects following periconception raltegravir exposure.
The analysis focused on prospective raltegravir exposures--those received before the pregnancy outcome was known. Merck researchers collected data from three sources: prospective pregnancy outcomes reported to the Merck safety database and two published European sources not included in the Merck database--the National Study of HIV in Pregnancy and Childhood (NSHPC) in the United Kingdom and Ireland and the ANRS-French Perinatal Cohort.
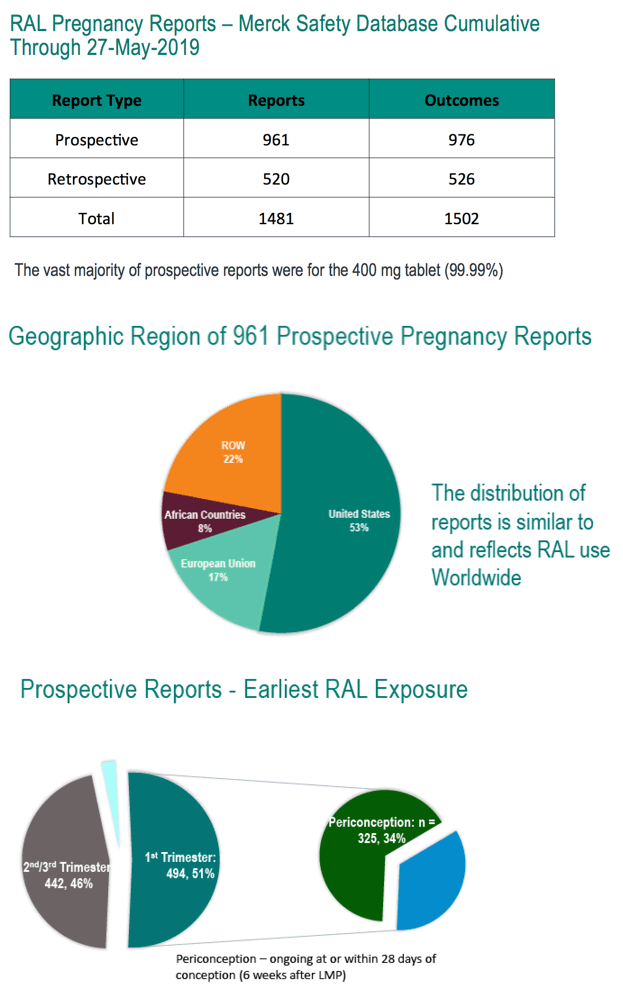
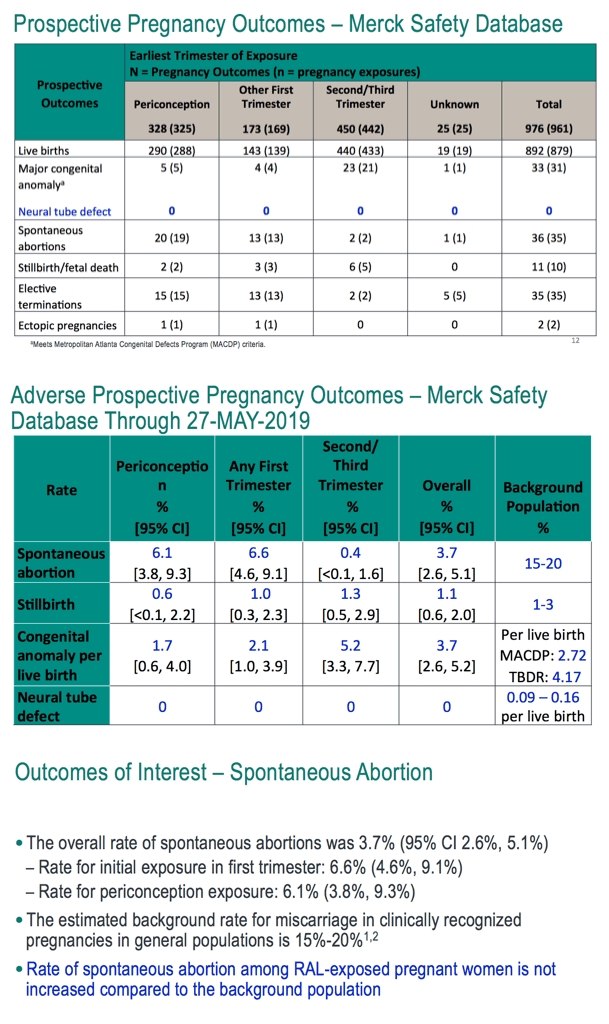
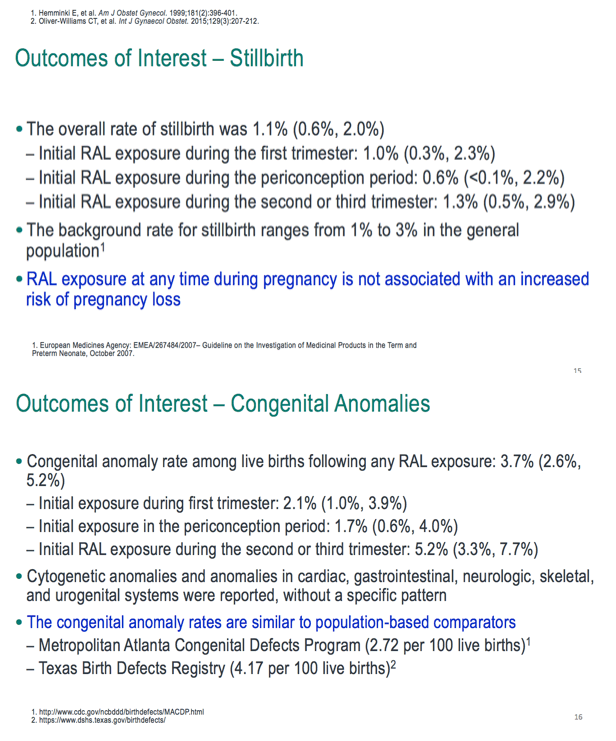
Through May 27, 2019, the Merck safety database included 961 prospective reports with 976 outcomes, about half of them from the United States, about half dating from the first trimester and one third involving periconception exposure. Among the 976 pregnancy outcomes in the Merck database, there were 892 live births, 33 major congenital anomalies (no neural tube defects), 36 spontaneous abortions, 11 stillbirths or fetal deaths, 35 elective terminations, and 2 ectopic pregnancies. Rates of congenital anomalies per live birth, spontaneous abortion, and stillbirths were similar to those in the US general population.
The UK/Ireland NSHPC database involved 886 raltegravir exposures during pregnancy from 2008 through 2018, including 256 in the first trimester, of which 222 involved exposure to raltegravir at the time of conception. The overall congenital anomaly rate was 2.59%, and the rate for exposure at conception was 2.25%. The NSHPC recorded no neural tube defects.
The ANRS-French Perinatal Cohort saw no evidence of a higher birth defect rate among 218 women taking raltegravir at conception. And the French reported no neural tube defects with raltegravir.
The Merck team concluded that these three large databases (focused largely on the United States and Western Europe) produced no data suggesting an association between raltegravir during any stage of pregnancy and adverse birth outcomes. Rates of spontaneous abortions, stillbirths, and major congenital anomalies reflected general-population rates in the regions studied. The 765 recorded periconception raltegravir exposures led to no neural tube defects.
Reference
1. Shamsuddin H, Raudenbush C, Sciba B, et al. Pregnancy outcomes following raltegravir exposure. IDWeek, October 2-6, 2019, Washington, DC. Abstract 886.


|
| |
|
 |
 |
|
|