 |
 |
 |
| |
Every-2-Month Maintenance CAB + RPV Noninferior to Monthly Dosing for 48 Weeks. ATLAS-2M study
|
| |
| |
CROI 2020, March 8-11, 2020, Boston
Mark Mascolini
Long-acting every-2-month cabotegravir (CAB) plus rilpivirine (RPV) proved virologically noninferior to monthly CAB + RPV in a randomized open-label trial involving over 1000 people who already had an undetectable viral load taking monthly CAB + RPV or another regimen [1]. Researchers counted 8 confirmed virologic failures (sequential viral loads above 200 copies) in the every-2-month arm versus 2 in the monthly arm.
The randomized ATLAS [2] and FLAIR [3] phase 3 trials found every-4-week injected CAB + RPV noninferior to daily oral three-drug antiretroviral therapy in maintaining virologic suppression. The phase 2 LATTE-2 trial [4] also studied every-8-week CAB + RPV.
To further compare CAB + RPV monthly and every 2 months, ATLAS-2M researchers recruited 391 people with sustained viral suppression in the original ATLAS trial of every-4-week CAB + RPV and 654 people with a sustained viral load below 50 copies while taking a protease inhibitor, a nonnucleoside, or an integrase inhibitor with two nucleosides/nucleotides. Participants not coming from ATLAS took oral CAB + RPV for 4 weeks before starting long-acting injections. In a 96-week maintenance phase, researchers randomized people 1-to-1 to injected CAB + RPV every 4 weeks or 8 weeks.
Almost two thirds of participants in each study arm (63%) had never taken CAB + RPV. Median age stood at 42 in each group, and 27% in each group were 50 or older. Just over one quarter of participants in each group were female at birth. Whites made up about three quarters of each study arm, and blacks comprised just under 20% of each arm. Median overall CD4 count stood at 661.
After 48 weeks the proportion of people with a viral load at or above 50 copies (the primary endpoint) in a snapshot (intention-to-treat exposed) analysis measured 1.7% in the 8-week arm and 1% in the 4-week arm, The difference of 0.8% lay well within the 4% margin establishing every-8-week dosing as noninferior to every-4-week dosing. At 48 weeks 94.3% in the 8-week group and 93.5% in the 4-week group had a viral load below 50 copies. Respective proportions who discontinued because of adverse events or death were 1.7% and 2.5% and who discontinued for other reasons 2.3% and 3.1%.
The ATLAS-2M team tallied 8 confirmed virologic failures in the 8-week arm (1.5%) and 2 (0.4%) in the 4-week arm. Six of 8 people with confirmed failure in the 8-week group had treatment-emergent RPV resistance mutations, and 5 of 8 had emergent integrase inhibitor mutations. Of the 2 people with confirmed virologic failure in the 4-week arm, 1 had treatment-emergent RPV resistance mutations, and 2 had treatment-emergent integrase inhibitor mutations.
Comparing the every-8-week group with the every-4-week group, the researchers found similar rates of drug-related adverse events (21% and 24%), any grade 3 or worse adverse event (6% and 6%), any drug-related grade 3 or worse event (under 1% in both arms), and drug-related serious adverse events (under 1% in both arms). The number of drug-related adverse events leading to withdrawal came to 5 (less than 1%) in the 8-week group and 8 (2%) in the 4-week group. Problems leading to withdrawal were injection site reactions, fatigue, excess sweating, and abnormal dreams. Grade 3 or worse injection site reactions affected 2% in each study arm. These reactions led to 6 withdrawals (1%) in the 8-week group and 11 (2%) in the 4-week group.
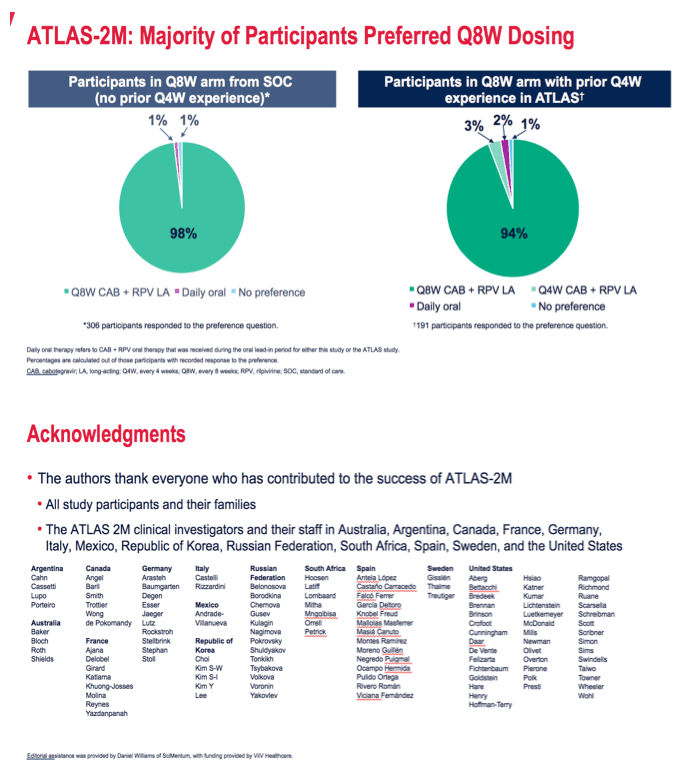
Among trial participants randomized to every-8-week therapy with no prior CAB + RPV experience, 98% said they preferred an injection every 8 weeks, 1% preferred daily oral dosing, and 1% had no preference. Among participants who with CAB + RPV experience, 94% preferred getting an injection every 8 weeks versus every 4.
References
1. Overton ET, Richmond GJ, Rizzardini G, et al. Cabotegravir + rilpivirine every 2 months is noninferior to monthly: ATLAS-2M study. Conference on Retroviruses and Opportunistic Infections (CROI). March 8-11, 2020. Boston. Abstract 34.
2. Swindells S, Andrade-Villanueva JF, Richmond GJ, et al. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N Engl J Med. 2020 Mar 4. doi: 10.1056/NEJMoa1904398.
3. Orkin C, Arasteh K, Hernandez-Mora MG, et al. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N Engl J Med. 2020 Mar 4. doi: 10.1056/NEJMoa1909512.
4. Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet. 2017;390:1499-1510.

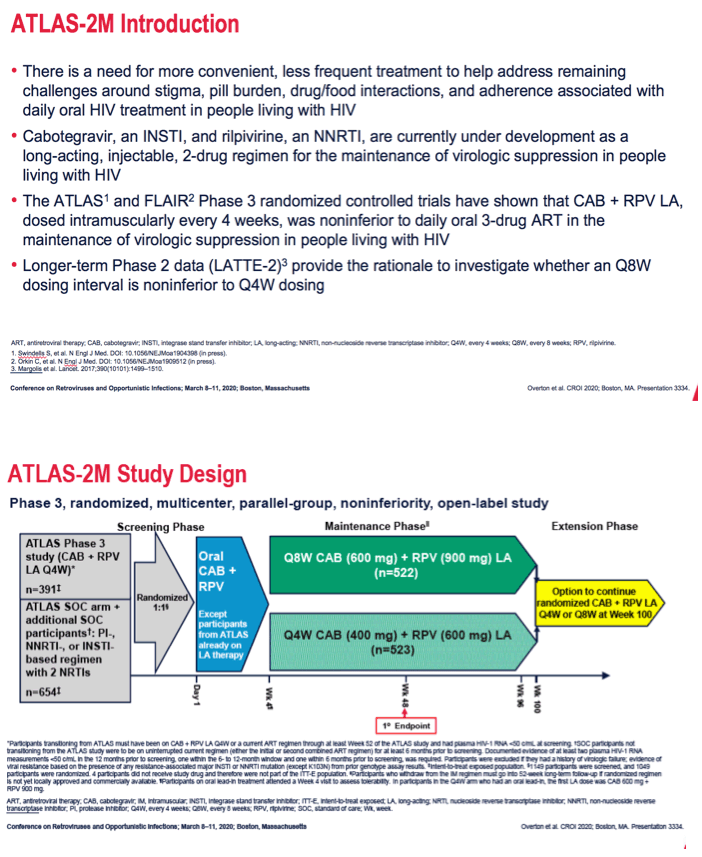
*Participants transitioning from ATLAS must have been on CAB + RPV LA Q4W or a current ART regimen through at least Week 52 of the ATLAS study and had plasma HIV-1 RNA <50 c/mL at screening. SOC participants not transitioning from the ATLAS study were to be on uninterrupted current regimen (either the initial or second combined ART regimen) for at least 6 months prior to screening. Documented evidence of at least two plasma HIV-1 RNA measurements <50 c/mL in the 12 months prior to screening, one within the 6- to 12-month window and one within 6 months prior to screening, was required. Participants were excluded if they had a history of virologic failure; evidence of viral resistance based on the presence of any resistance-associated major INSTI or NNRTI mutation (except K103N) from prior genotype assay results. Intent-to-treat exposed population. 1149 participants were screened, and 1049 participants were randomized. 4 participants did not receive study drug and therefore were not part of the ITT-E population. ǁParticipants who withdraw from the IM regimen must go into 52-week long-term follow-up if randomized regimen is not yet locally approved and commercially available. Participants on oral lead-in treatment attended a Week 4 visit to assess tolerability. In participants in the Q4W arm who had an oral lead-in, the first LA dose was CAB 600 mg +
RPV 900 mg.
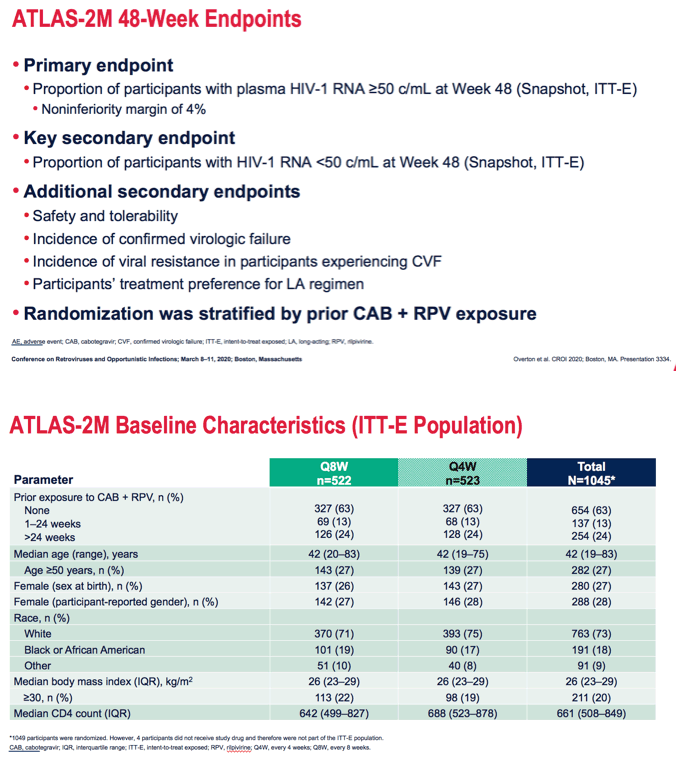
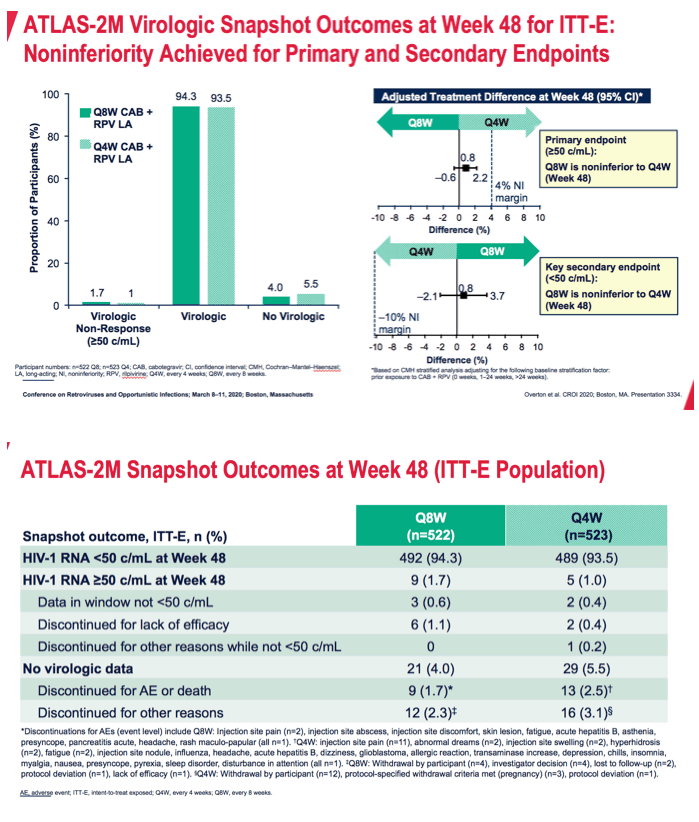
*Discontinuations for AEs (event level) include Q8W: Injection site pain (n=2), injection site abscess, injection site discomfort, skin lesion, fatigue, acute hepatitis B, asthenia, presyncope, pancreatitis acute, headache, rash maculo-papular (all n=1). Q4W: injection site pain (n=11), abnormal dreams (n=2), injection site swelling (n=2), hyperhidrosis (n=2), fatigue (n=2), injection site nodule, influenza, headache, acute hepatitis B, dizziness, glioblastoma, allergic reaction, transaminase increase, depression, chills, insomnia, myalgia, nausea, presyncope, pyrexia, sleep disorder, disturbance in attention (all n=1). Q8W: Withdrawal by participant (n=4), investigator decision (n=4), lost to follow-up (n=2), protocol deviation (n=1), lack of efficacy (n=1). Q4W: Withdrawal by participant (n=12), protocol-specified withdrawal criteria met (pregnancy) (n=3), protocol deviation (n=1).
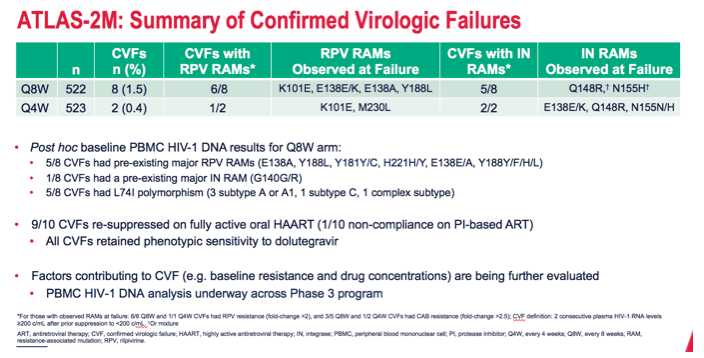
*For those with observed RAMs at failure: 6/6 Q8W and 1/1 Q4W CVFs had RPV resistance (fold-change >2), and 3/5 Q8W and 1/2 Q4W CVFs had CAB resistance (fold-change >2.5); CVF definition: 2 consecutive plasma HIV-1 RNA levels ≥200 c/mL after prior suppression to <200 c/mL. Or mixture
ART, antiretroviral therapy; CVF, confirmed virologic failure; HAART, highly active antiretroviral therapy; IN, integrase; PBMC, peripheral blood mononuclear cell; PI, protease inhibitor; Q4W, every 4 weeks; Q8W, every 8 weeks; RAM, resistance-associated mutation; RPV, rilpivirine
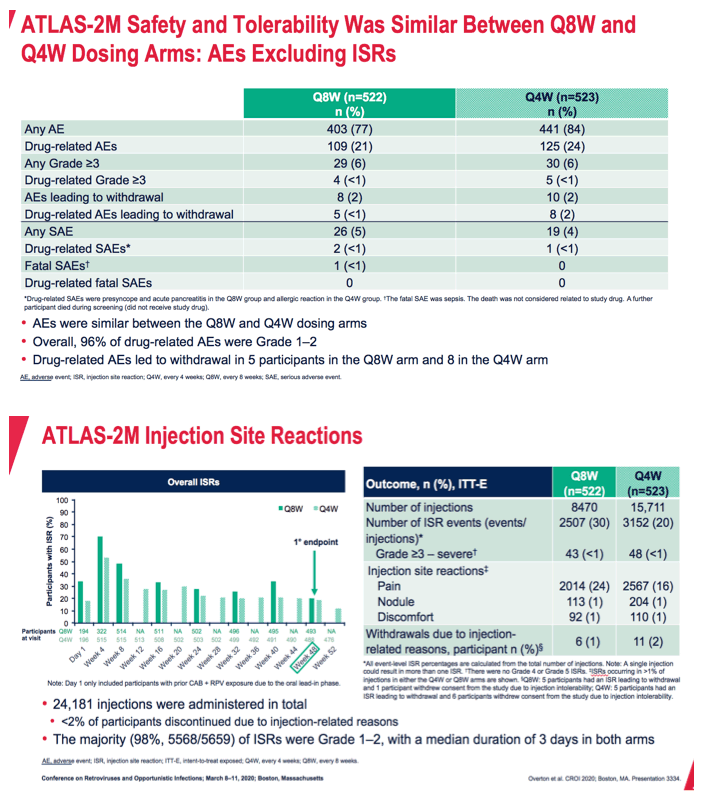
*All event-level ISR percentages are calculated from the total number of injections. Note: A single injection could result in more than one ISR. There were no Grade 4 or Grade 5 ISRs. ISRs occurring in >1% of injections in either the Q4W or Q8W arms are shown. Q8W: 5 participants had an ISR leading to withdrawal and 1 participant withdrew consent from the study due to injection intolerability; Q4W: 5 participants had an ISR leading to withdrawal and 6 participants withdrew consent from the study due to injection intolerability
AE, adverse event; ISR, injection site reaction; ITT-E, intent-to-treat exposed; Q4W, every 4 weeks; Q8W, every 8 weeks
|
| |
|
 |
 |
|
|