 |
 |
 |
| |
CLINICAL AND LABORATORY OUTCOMES 24 WEEKS AFTER STARTING DTG VERSUS EFV IN ACUTE HIV
|
| |
| |
CROI 2020
Reported by Jules Levin
Phillip Chan1, Orlanda Goh1, Donn J. Colby1, Carlo Sacdalan1, Camilla Muccini2, Nittaya Phanuphak1, Suteeraporn Pinyakorn3, Praphan Phanuphak4, Nitiya Chomchey1
1SEARCH, Bangkok, Thailand,2San Raffaele Vita-Salute University, Milan, Italy,3US Military HIV Research Program, Bethesda, MD, USA,4Thai Red Cross AIDS Research Center, Bangkok, Thailand,5University of Missouri St Louis, St Louis, MO, USA,6Yale University, New Haven, CT, USA
Abstract
This study compared clinical and laboratory parameters before and after initiating Efavirenz(EFV)- and Dolutegravir(DTG)-based antiretroviral therapy (ART), the prior and current 1st line ART, during acute HIV infection (AHI).
Individuals with AHI (Fiebig I-V) enrolled in the RV254 cohort in Thailand initiated ART within days (median=0; IQR 0-1) after diagnosis (EFV+2 NRTI: 2009-Jan2017; DTG+2 NRTI: Feb2017 onwards). Plasma HIV-1 RNA, blood CD4 and CD8 T-cell counts, and mood parameters, measured by the 9-item Patient Health Questionnaire (PHQ-9, score 0-27) for depression symptoms and the Distress Thermometer (DT) for anxiety/stress (score 0-10) were measured before and 24 weeks after ART. Participants who received other ART regimens were excluded.
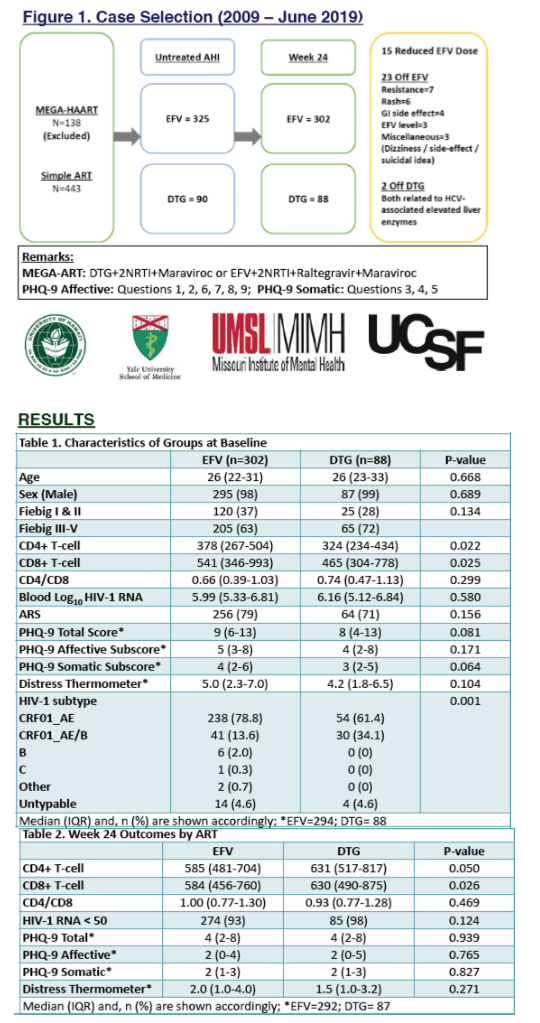
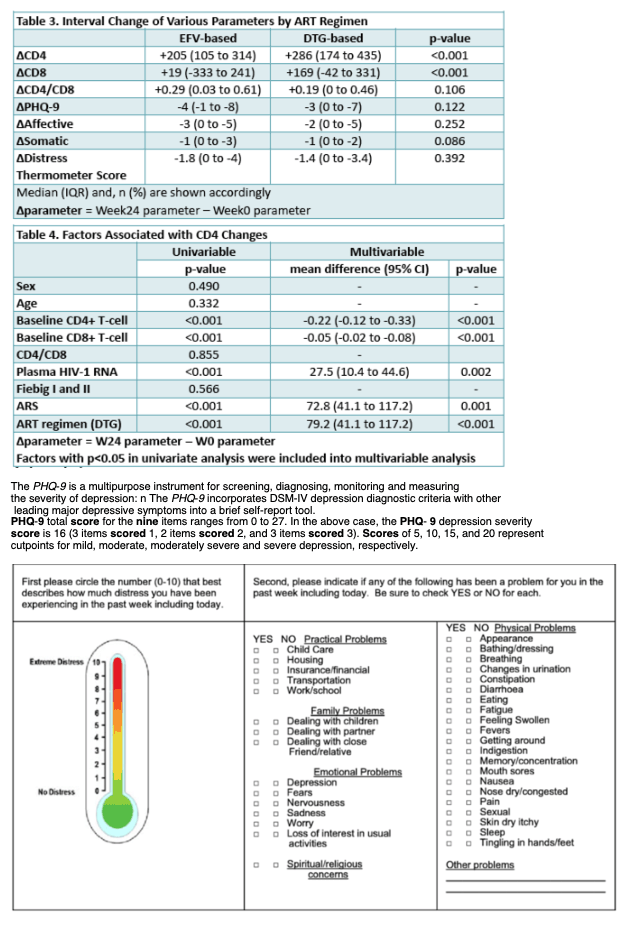
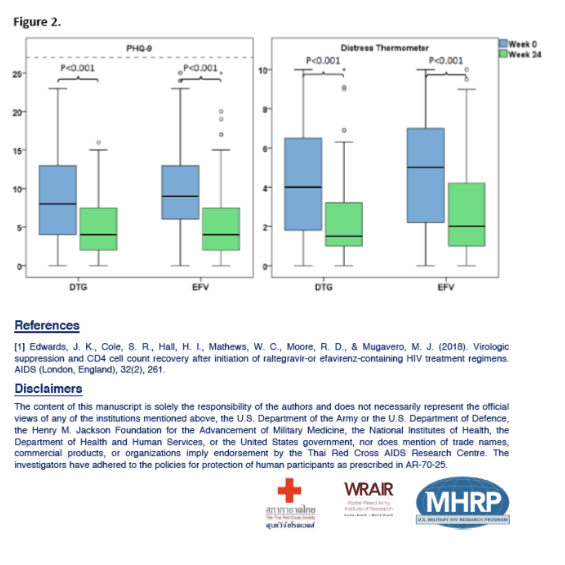
From 2009-2019, 415 participants (98% male, median age 26 years) initiated ART at AHI (EFV-based=325; DTG-based=90). By week 24, 15 (5%) EFV users reduced their daily EFV dose from 600mg to 300mg due to side effects and super-therapeutic plasma EFV levels. Another 23 (7%) discontinued EFV due to EFV-associated adverse events (AEs) and/or resistance; 2 (2%) DTG users discontinued DTG, both for acute hepatitis C with liver enzyme elevations (p=0.130). At baseline, both groups (EFV=302; DTG=88) were similar in age, sex composition, CD4/CD8 ratio, plasma HIV-1 RNA, PHQ-9 and DT scores (p>0.05); 167 (43%) had moderate depression symptoms (PHQ-9>9). The DTG group had lower CD4 and CD8 T-cells and higher rates of Fiebig III and CRF01 AE/B recombinant subtype than the EFV group (p<0.05). HIV suppression (<50 copies/ml) rates were 98% and 93% in the DTG and EFV group respectively (p=0.124). Comparing the change of parameters (i.e. difference between week 24 and baseline) by ART regimen showed greater gain in CD4 and CD8 T-cells in DTG users (Table). DTG-based ART remained independently associated with greater CD4 recovery (mean diff +78.0, 95%CI [40.2 to 115.8], p<0.001) in multivariable analysis. At week 24, the rate of PHQ-9>9 in the DTG and EFV groups were 15% vs 13% respectively (p=0.644). Both groups had lower PHQ-9 and DT scores than at baseline (p<0.001) but both scores were similar across the groups (p>0.05).
Compared to EFV, initiating DTG-based ART at AHI was associated with a greater gain in CD4 T-cells and a higher absolute CD4 count at week 24. There were no DTG related AEs leading to discontinuation. Self-reported depression symptoms observed at AHI improved with ART regardless of the regime.

|
| |
|
 |
 |
|
|