 |
 |
 |
| |
NEWBORN TESTING REVEALS HIGH HCV SEROPREVALENCE IN PREGNANT WOMEN FROM NEW YORK STATE
|
| |
| |
program abstract
Linda M.Styer1, Erica Miller2, Jean Rock1, Lea Krein1, Monica Martin1, Dhanushki Samaranayake1, Shu-Yin Leung1, Michele Caggana1, Colleen Flanigan1, Monica Parker1
1New York State Department of Health, Albany, NY, USA, 2University at Albany, Albany, NY, USA
Background: Hepatitis C virus (HCV) infections in New York State (NYS) have been rising among young adults due to increased injection drug use. In 2018 in NYS (excluding NYC), 61% of new female cases were in women of child bearing age (15-44 yrs old). Increased HCV infections in this age group are concerning as 6% of HCV RNA-positive pregnant women will transmit HCV to their baby. To plan effective public health actions, accurate HCV prevalence rates among pregnant women are needed; however, many HCV infections go undiagnosed and unreported. Babies passively acquire maternal IgG antibodies. Therefore, testing newborn blood for HCV antibodies can reveal mom’s serostatus. Our goal was to perform a large-scale HCV serosurvey of pregnant women in NYS by testing newborn dried blood spots (DBS) using a high-throughput, low-cost Luminex HCV immunoassay.
Methods: All DBS submitted to NYS’s newborn screening program over 6 wks were sampled by punching a 3mm circle into microplates. Aggregate data on birth weight, gestational age and mother’s county of residence were recorded, and samples were blinded. A generic patient code was included to identify duplicate samples. HCV antigen-coupled beads were used to test eluted blood for HCV antibodies using a low-cost (<$0.80/well) Luminex-based immunoassay in 384-well plates. Repeated median fluorescence intensity (MFI) >1000 was considered HCV antibody reactive.
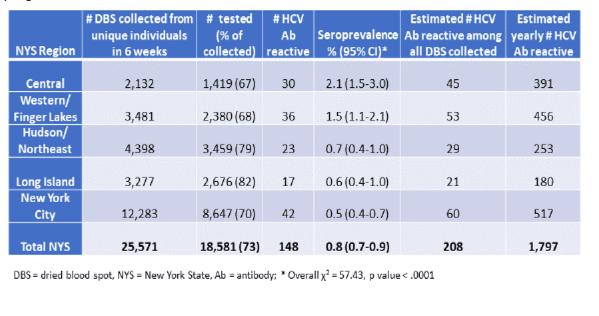
Results: Of the 29,323 DBS sampled, 25,571 (87%) were from unique babies born to mothers residing in NYS. Of these, 18,581 (73%) were tested. 148 DBS were HCV antibody reactive, for an overall NYS seroprevalence of 0.8%. Multiple DBS collected on different days were tested from 1409 individuals, 31 with repeat HCV reactive results, 1376 with repeat non-reactive results and 2 with discordant results close to the MFI cutoff. Premature birth (26%) and low birth weight (26%) were twice as common in babies born to HCV seropositive mothers than seronegative mothers (p<0.001). HCV seroprevalence in Central (2.1%) and Western/Finger Lakes (1.5%) regions, where multiple counties are designated rural, was 3-4 times higher than the rest of NYS and similar to high rates observed in other U.S. rural regions. For the year, we estimate that ~1800 babies will be born to HCV antibody positive women in NYS.
Conclusion: Newborn DBS testing using a Luminex-based immunoassay is an effective way to assess HCV burden among pregnant women.
|
| |
|
 |
 |
|
|