 |
 |
 |
| |
DISCOVER: NO EFFECT OF HORMONES ON F/TAF OR F/TDF PK, EFFICACY & SAFETY IN TRANSWOMEN
|
| |
| |
CROI 2020 March 8-11
Reported by Jules Levin
Michelle S. Cespedes1, Sophia R. Majeed2, Maria Prins3, Ivanka Krznaric4, Anita Mathias2, Deqing Xiao2, Pamela Wong2, Jason Hindman2, Christoph Carter2, Dia
1Icahn School of Medicine at Mount Sinai, New York, NY, USA,2Gilead Sciences, Inc, Foster City, CA, USA,3University of Amsterdam, Amsterdam, Netherlands,4Center for Infectious Diseases, Berlin, Germany,5Public Health Service Amsterdam, Amsterdam, Netherlands,6Peter J Ruane MD Inc, Los Angeles, CA, USA,7Huntridge Family Clinic, Las Vegas, NV, USA,8CrescentCare, New Orleans, LA, USA
Program Abstract
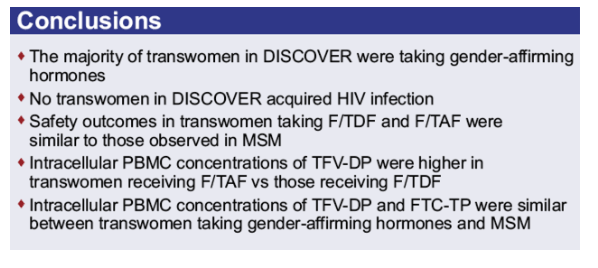
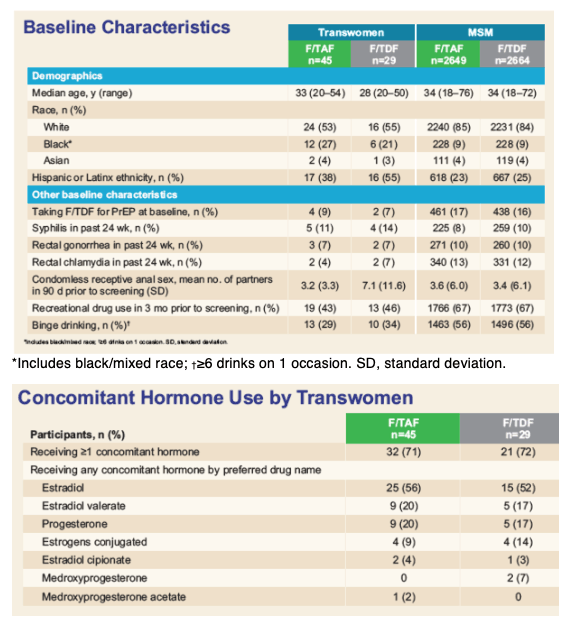
Emtricitabine (F), tenofovir alafenamide (TAF) and tenofovir disoproxil fumarate (TDF) do not have relevant drug interactions with low-dose hormones used for contraception. The current analysis explored PK as well as efficacy and safety of transwomen receiving either F/TAF or F/TDF in the DISCOVER trial, the majority of whom were taking high-dose, gender-affirming, hormonal therapy.
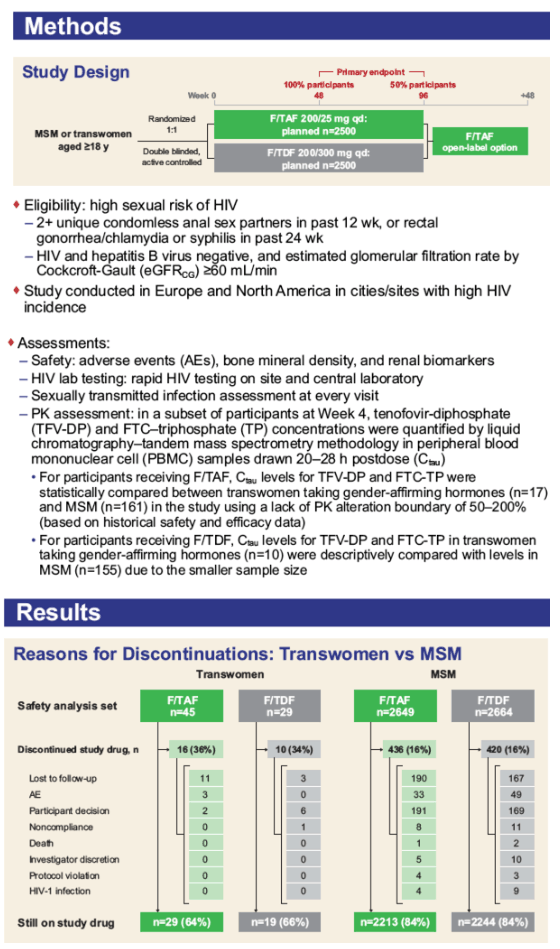
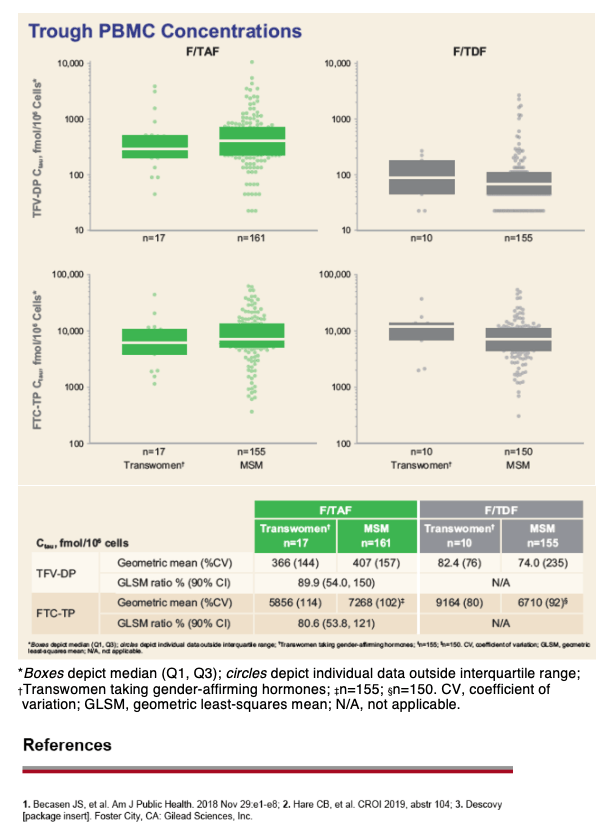
Overall, 74 transwomen at risk of HIV were randomized 1:1 to receive blinded F/TAF or F/TDF once daily in DISCOVER. Efficacy and safety results are summarized. TFV-DP and FTC-TP PBMC trough levels (Ctau; defined as 20 to 28 hours postdose) were evaluated at steady-state (W4) and compared between transwomen taking high-dose hormones concomitantly with F/TAF (N=17) and a randomly pre-selected, representative group of MSM randomized to F/TAF not using high-dose hormones (N=161) using geometric least squares mean (GLSM) ratios and 90% confidence intervals (CIs). Comparisons were made using a lack of PK alteration boundary of 50 to 200% to identify potentially clinically relevant differences. Levels of TFV-DP and FTC-TP with F/TDF in transwomen on high-dose hormones (N=10) were compared descriptively to levels in MSM randomized to F/TDF (N=155) due to a smaller sample size.
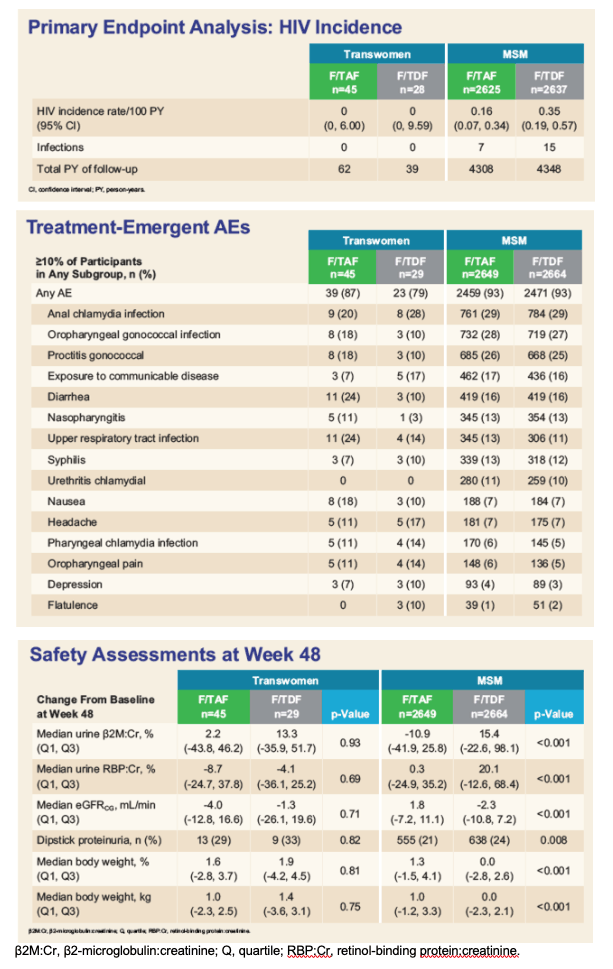
No transwomen acquired HIV. Transwomen had similar numerical improvements in dipstick proteinuria and markers of tubular proteinuria as MSM. No transwomen developed clinically significant proteinuria (UPCR>200 mg/g). There were no differences between F/TAF and F/TDF in change from baseline in weight or eGFR in transwomen. GLSM ratios and 90% CIs for comparisons of PBMC TFV-DP and FTC-TP levels with F/TAF between transwomen taking high-dose hormones and MSM were within the 50 to 200% boundary, indicating no clinically significant interaction. In transwomen taking F/TDF and high-dose hormones, TFV-DP and FTC-TP levels were comparable to MSM, suggesting no interaction (Table).
The absence of infections suggests that both F/TAF and F/TDF were effective for HIV prevention in transwomen. Both F/TAF and F/TDF were safe and well-tolerated in transwomen and MSM. No clinically meaningful differences in PBMC TFV-DP and FTC-TP levels with F/TAF or F/TDF were observed between transwomen taking high-dose hormone therapy and MSM, suggesting that both F/TAF and F/TDF are effective and safe options for PrEP in transwomen on gender-affirming, high-dose hormone therapy.

|
| |
|
 |
 |
|
|