| |
|
Short-Duration Pan-Genotypic Therapy With Glecaprevir/Pibrentasvir for 6 Weeks Among People With Recent Hepatitis C Viral Infection
|
| |
| |
April 8 2020 Hepatology - Marianne Martinello ,1-3 Chloe Orkin,4 Graham Cooke,5 Sanjay Bhagani,6 Edward Gane,7 Ranjababu Kulasegaram,8 David Shaw,9 Elise Tu,1 Kathy Petoumenos,1 Philippa Marks,1 Jason Grebely,1 Gregory J. Dore,1,2 Mark Nelson,10* and Gail V. Matthews1,2*
Download the PDF here
Glecaprevir/pibrentasvir for 6 weeks was highly effective, safe, and well tolerated among people with acute and recent HCV infection (SVR12 ITT 90%, SVR12 PP 96%), including among people with HIV coinfection and high baseline HCV RNA (>6 log10 IU/mL). Treatment resulted in rapid HCV RNA suppression and normalization of liver enzymes. No treatment-related serious adverse events were reported.
In order to achieve HCV elimination targets,28 increased diagnosis and treatment of recent HCV infection will be required.29 Striving for microelimination in high-incidence populations is an important step toward reaching HCV elimination targets. High and increasing HCV incidence rates among populations of PWID and HIV-positive MSM highlight the need to determine the optimal duration of therapy and choice of DAA regimen in recent infection, including for treatment of reinfection. Access to HCV care and treatment for people at high risk of onward transmission, including those with recent HCV infection, should be a priority.1 HCV treatment-as-prevention efforts should be enhanced by the immediate commencement of DAA therapy in people with recent HCV. A targeted "test and treat" (and retreat) strategy, with short-duration DAA therapy among at-risk populations, may be one of the most cost-effective public health strategies in attempts to eliminate HCV.30
HIV coinfection was documented in 77% (n = 23); median CD4 count was 571 x 106/L (range 341-1,488). All HIV-positive participants were receiving combination antiretroviral therapy (n = 23, 100%), with HIV RNA ≤ 50 copies/mL in 96% (n = 22). At baseline, most (n = 18, 78%) were receiving an INSTI (dolutegravir or raltegravir) plus two nucleoside reverse transcriptase inhibitors (Supporting Table S2). Four (13%) participants required alterations to their antiretroviral regimen given potential drug interactions; all changed from a non-nucleoside reverse transcriptase inhibitor (n = 2) or boosted-protease inhibitor (n = 2) to an INSTI. Of the three HIV-negative MSM enrolled, one was receiving HIV preexposure prophylaxis.
Fourteen (47%) participants had ever injected drugs, with 9 (30%) reporting injecting drug use within 6 months of enrollment. (Meth)amphetamine use, ever and current, was predominant, by both injecting (ever, 40%; current, 30%) and noninjecting (ever, 60%; current, 33%) routes of administration. Indeed, (meth)amphetamine was the only drug injected in the 6 months prior to enrollment (Supporting Table S3). Among participants who reported injecting drug use, median age at first injecting was 37 years (range 19-55). Median duration of injecting drug use prior to estimated date of HCV infection was 2.1 years (IQR 1.0-3.6). Only 2 participants (7%) had ever received opioid substitution therapy, with no participants on such therapy at enrollment.
Abstract
Background and Aims
Among treatment-naive individuals with chronic hepatitis C viral (HCV) infection and without cirrhosis, glecaprevir/pibrentasvir for 8 weeks is recommended. The aim of this analysis was to evaluate the efficacy of glecaprevir/pibrentasvir for 6 weeks in people with acute and recent HCV infection.
Approach and Results
In this open-label, single-arm, multicenter, international pilot study, adults with recent HCV (duration of infection < 12 months) received glecaprevir/pibrentasvir 300/120 mg daily for 6 weeks. Primary infection was defined by first positive anti-HCV antibody and/or HCV RNA within 6 months of enrollment and either acute clinical hepatitis within the past 12 months (symptomatic seroconversion illness or alanine aminotransferase > 10 x upper limit of normal) or anti-HCV antibody seroconversion within 18 months. Reinfection was defined as new positive HCV RNA within 6 months of enrollment and evidence of prior spontaneous or treatment-induced clearance. The primary endpoint was sustained virologic response at 12 weeks posttreatment (SVR12). Thirty men (median age 43 years, 90% men who have sex with men) received treatment, of whom 77% (n = 23) were human immunodeficiency virus–positive, 47% (n = 14) had ever injected drugs, and 13% (n = 4) had HCV reinfection. The majority had HCV genotype 1 (83%, n = 25), followed by genotype 4 (10%, n = 3) and genotype 3 (7%, n = 2).
At baseline, median estimated duration of infection was 29 weeks (range 13, 52) and median HCV RNA was 6.2 log10 IU/mL (range 0.9, 7.7).
SVR12 in the intention-to-treat and per-protocol populations was achieved in 90% (27/30) and 96% (27/28), respectively. There was one case of relapse, and there were two cases of nonvirological failure (death, n = 1; loss to follow-up, n = 1). No treatment-related serious adverse events were seen.
Conclusions
Glecaprevir/pibrentasvir for 6 weeks was highly effective among people with acute and recent HCV infection, supporting further evaluation of shortened-duration pan-genotypic therapy in this setting.
Treatment Adherence and Outcomes
Adherence to therapy was high, with all participants completing the 6-week course of therapy. By pill count and self-report, adherence > 95% was 100%, with median on-treatment adherence 100%.
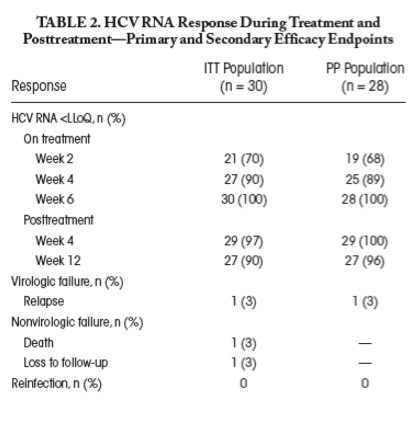
In the ITT population, SVR12 was achieved in 90% (27/30; 95% CI 73%, 98%) (Fig. 2 and Table 2). In the PP population, SVR12 was 96% (27/28; 95% CI 82%, 100%) (for efficacy by genotype, see Supporting Fig. S1). Among participants with HIV coinfection, SVR12 in the ITT and PP populations was 87% (20/23; 95% CI, 66-97%) and 95% (20/21; 95% CI, 76-100%), respectively. Among participants with HCV monoinfection, SVR12 in the ITT and PP populations was 100% (7/7; 95% CI, 59-100%) and 100% (7/7; 95% CI, 59-100%). Among participants with baseline HCV RNA > 6 log10 IU/mL, SVR12 in the ITT and PP populations was 88% (15/17; 95% CI, 64-99%) and 94% (15/16; 95% CI, 70-100%), respectively (Fig. 3).
Virological suppression at end of treatment was documented in 100% (30/30; 95% CI, 88-100%) (Fig. 2). At weeks 2, 4, and 6, 70%, 90%, and 100% had HCV RNA below the LLoQ, with 22%, 70%, and 93% having HCV RNA below the lower limit of detection, respectively (Table 2; Supporting Fig. S2). Two participants with detectable HCV RNA (HCV RNA < 10, not quantifiable) at week 6 (end of treatment) achieved SVR12 (HCV RNA target not detected). A rapid biochemical response on treatment was observed (Fig. 4); median ALT values at baseline and week 6 (end of treatment) were 203 U/L (range 30-707) and 22 U/L (range 12-77) (P < 0.001) (Supporting Table S4).
Of those participants who did not achieve SVR12 (n = 3), there was one case of virological failure and there were two cases of nonvirological failure. In the cases of nonvirological failure, 1 participant died after posttreatment week 4 (achieved SVR4; HCV RNA target not detected at last study contact) and 1 participant was lost to follow-up after end of treatment (achieved end-of-treatment response; HCV RNA target not detected at last study contact). Virological failure, confirmed as relapse on sequencing, was observed in 1 (3%) participant with acute genotype 1a HCV infection (Fig. 5; Supporting Fig. S3). The participant was a 50-year-old man with HIV infection, who was diagnosed with primary acute HCV in the setting of asymptomatic seroconversion. At screening and baseline, estimated duration of HCV infection was 18 and 24 weeks, respectively, with a narrow seroconversion window as the last negative anti-HCV antibody was only 5 weeks prior to the first positive anti-HCV antibody. Baseline HCV RNA was 7.7 log10 IU/mL. The participant was adherent to treatment, and HCV RNA declined rapidly (week 2, HCV RNA 38 IU/mL [1.6 log10 IU/mL]; week 4, HCV RNA <10 IU/mL [target detected, not quantifiable]; week 6, HCV RNA target not detected). Recurrence of HCV viremia was identified at week 12 posttreatment (posttreatment week 4, HCV RNA <10 IU/mL [target detected, not quantifiable]; posttreatment week 12, HCV RNA 7.5 log10 IU/mL). No significant NS3 or NS5A resistance-associated polymorphisms were detected at baseline or posttreatment week 12. The participant received retreatment with sofosbuvir/velpatasvir/voxilaprevir for 12 weeks and achieved SVR12.
Results
Participant Disposition and Overview of the Study Population
Between October 31, 2017, and August 7, 2018, 39 individuals were screened and 30 enrolled (Fig. 1). All enrolled participants were male (n = 30, 100%), most of whom identified as MSM (n = 27, 90%). The majority were infected with HCV genotype 1 (n = 25, 83%; 1a, n = 22, 73%; 1b, n = 1, 3%; 1, no subtype, n = 2, 7%), followed by genotype 4 (n = 3, 10%) and genotype 3 (n = 2, 7%) (Table 1). Recent primary HCV infection was documented in 26 (87%) and recent HCV reinfection in 4 (13%); all participants with recent HCV reinfection had previously achieved SVR following treatment (Supporting Table S1). The predominant clinician-determined modes of HCV acquisition were sexual exposure among MSM (n = 22, 73%) and injecting drug use (n = 5, 17%) (Table 1). Median maximum ALT in the preceding 12 months was 381 IU/L (range 26-3,087). Acute clinical hepatitis with ALT > 10 x ULN was documented in 73% (n = 22). Six (20%) participants had a symptomatic seroconversion illness, including 5 (17%) with jaundice. At screening and baseline, median estimated duration of infection was 23 weeks (range 8-41) and 29 weeks (range 13-52), respectively. Median baseline HCV RNA was 6.2 log10 IU/mL (range 1.0-7.7), with baseline HCV RNA > 1,000,000 IU/mL (>6 log10) in 57% (n = 17) and > 10,000,000 IU/mL (>7 log10) in 27% (n = 8). Median baseline ALT was 203 U/L (range 30-707), with a median liver stiffness measurement (FibroScan) of 5.5 kPa (IQR 4.7-7.5).
HIV coinfection was documented in 77% (n = 23); median CD4 count was 571 x 106/L (range 341-1,488). All HIV-positive participants were receiving combination antiretroviral therapy (n = 23, 100%), with HIV RNA ≤ 50 copies/mL in 96% (n = 22). At baseline, most (n = 18, 78%) were receiving an INSTI (dolutegravir or raltegravir) plus two nucleoside reverse transcriptase inhibitors (Supporting Table S2). Four (13%) participants required alterations to their antiretroviral regimen given potential drug interactions; all changed from a non-nucleoside reverse transcriptase inhibitor (n = 2) or boosted-protease inhibitor (n = 2) to an INSTI. Of the three HIV-negative MSM enrolled, one was receiving HIV preexposure prophylaxis.
Fourteen (47%) participants had ever injected drugs, with 9 (30%) reporting injecting drug use within 6 months of enrollment. (Meth)amphetamine use, ever and current, was predominant, by both injecting (ever, 40%; current, 30%) and noninjecting (ever, 60%; current, 33%) routes of administration. Indeed, (meth)amphetamine was the only drug injected in the 6 months prior to enrollment (Supporting Table S3). Among participants who reported injecting drug use, median age at first injecting was 37 years (range 19-55). Median duration of injecting drug use prior to estimated date of HCV infection was 2.1 years (IQR 1.0-3.6). Only 2 participants (7%) had ever received opioid substitution therapy, with no participants on such therapy at enrollment.
| |
| |
| |
|
|
|