 |
 |
 |
| |
Comparative efficacy and safety of a combination therapy of dolutegravir and lamivudine vs 3-drug antiretroviral regimens in treatment-naïve HIV-1 infected patients at 96 weeks: A systematic review and network meta-analysis.
|
| |
| |
Presented at the 23rd International AIDS Conference, July 6-10, 2020, San Francisco, California.
Reported by Jules Levin

Abstract
BACKGROUND: Dolutegravir+lamivudine (DTG+3TC) has been shown to be comparable to guideline recommended 3-drug antiretroviral treatments (ARTs) in HIV up to week 48. The objective of this analyses was to compare the efficacy and safety of DTG+3TC to 3-drug ARTs up to 96 weeks (WK96).
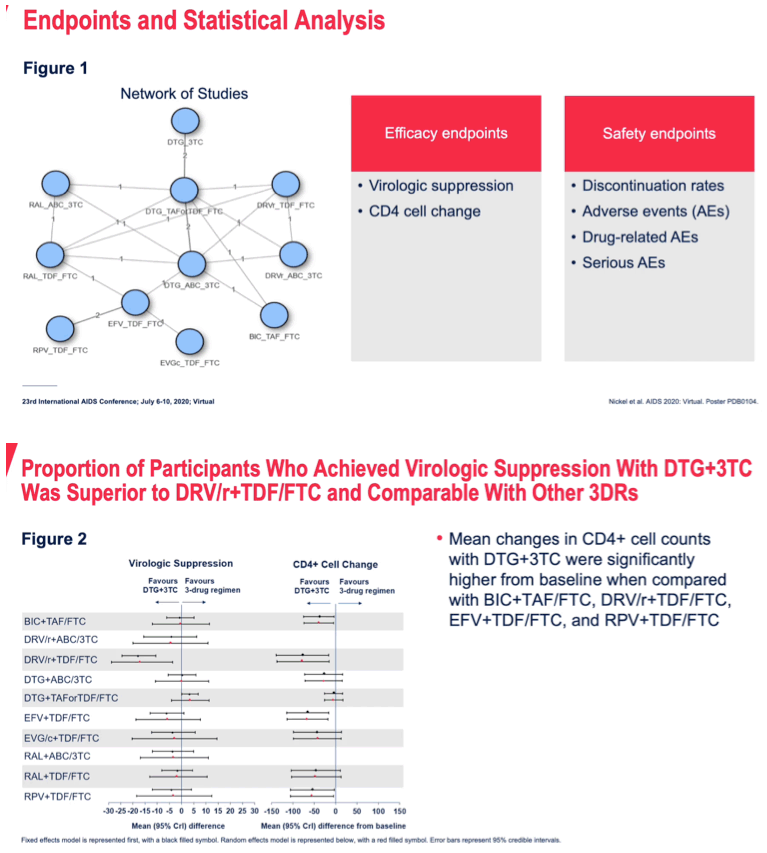
METHODS: Randomized controlled trials (RCTs) of treatment-naïve HIV-1 infected patients reporting outcomes at WK96 were identified by systematic review. The proportion of patients achieving virologic suppression (VS) to <50 RNA copies/mL at WK96 for all patients and those with baseline viral load >100,000 RNA copies/mL was compared between DTG+3TC and guideline recommended ARTs using a fixed-effect Bayesian network meta-analysis framework. Other outcomes examined were CD4+ cell count change from baseline, treatment discontinuations and safety (adverse events [AEs], serious AEs [SAEs], and drug-related AEs [DRAEs]).
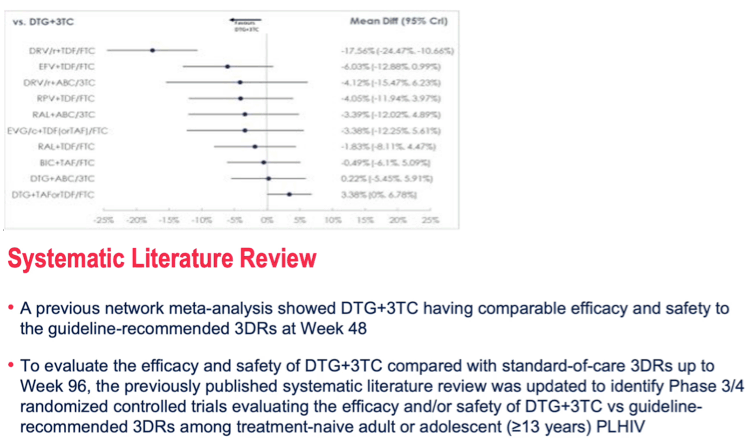
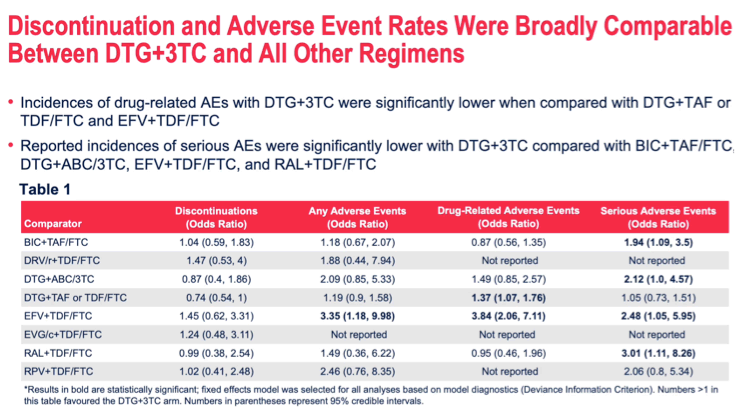
RESULTS: The network included 11 ARTs from 11 RCTs with 7991 patients. ARTs containing tenofovir disoproxil/emtricitabine (TDF/FTC) and tenofovir alafenamide (TAF)/FTC were combined by their core agent to maintain network connectivity. The treatment difference for viral suppression at WK96 for DTG+3TC compared to the other 10 ARTs ranged from -3% (-7%, 0%) vs DTG+TDF(or)TAF/FTC to 13% (4%, 23%) vs ritonavir-boosted darunavir (DRV/r)+TDF/FTC. On other outcomes DTG+3TC was broadly similar to all 3-drug ARTs with some statistically significant benefits on SAEs and DRAEs.
CONCLUSIONS: DTG+3TC offers comparable and durable efficacy and safety to guideline recommended 3-drug regimen with reduced exposure to ARTs for naïve patients starting treatment.
Figure 1: Difference in proportions in viral suppression (95% credible intervals) at WK96, 3-drug ARTs versus DTG+3TC.
|
| |
|
 |
 |
|
|