 |
 |
 |
| |
Concordance of the Xpert hepatitis B viral load test and conventional quantitative PCR in detecting and quantifying viremia using stored plasma and dried blood spot samples in West Africa
|
| |
| |
EASL 2022 June 22-26 London
Gibril Ndow1, Amie Ceesay1, Yusuke Shimakawa2, Alpha Omar Jallow1, Haddy Nyang1, Baboucarr Bittaye1, Francis Mendy1, Ousman Secka1, Umberto D'Alessandro1,
Mark Thursz3, Isabelle Chemin4, Maud Lemoine3. 1MRC Unit The Gambia at LSHTM, Disease Control and Elimination, Fajara, Gambia; 2Institut Pasteur, Unité d'épidémiologie des maladies émergentes, Paris, France; 3Imperial College London, Metabolism, Digestion and Reproduction, London, United Kingdom; 4INSERM U1052, Center de Recherche en Cancérologie, CNRS UMR5286, Lyon, France

program abstract
Background and aims: The new Xpert hepatitis B virus (HBV) viral load test (Cepheid, USA) provides an opportunity to scale up and decentralise hepatitis B diagnosis, treatment and monitoring in resource-limited settings where HBV is endemic and molecular diagnostics to quantify HBV DNA are scarce. We assessed the concordance between the new Xpert HBV viral load test and the conventional quantitative PCR (Abbott, USA) in quantifying HBV viremia using stored plasma and dried blood spot (DBS) samples.
Method: Paired plasma and DBS (Whatman 903 Protein Saver cards) samples were collected from adults with chronic HBV infection followed up in the PROLIFICA (Prevention of Liver Fibrosis and Cancer in Africa) programme in The Gambia. Samples were stored in - 80C for at least 12 months before analysis.
For each plasma sample, HBV DNAwas quantified using both realtime qPCR (Abbott, detection limit 26 IU/ml in plasma) and Xpert (detection limit 3.2 IU/ml in plasma). For DBS, HBV DNA was quantified using Xpert.
Concordance of the Xpert HBV test and the conventional qPCR in detecting and quantifying HBV viremia in plasma was assessed by kappa statistics, and the agreement between the two assays was assessed using the Bland-Altman plot. The concordance of HBV viremia between paired plasma and DBS samples was also assessed by kappa statistics.
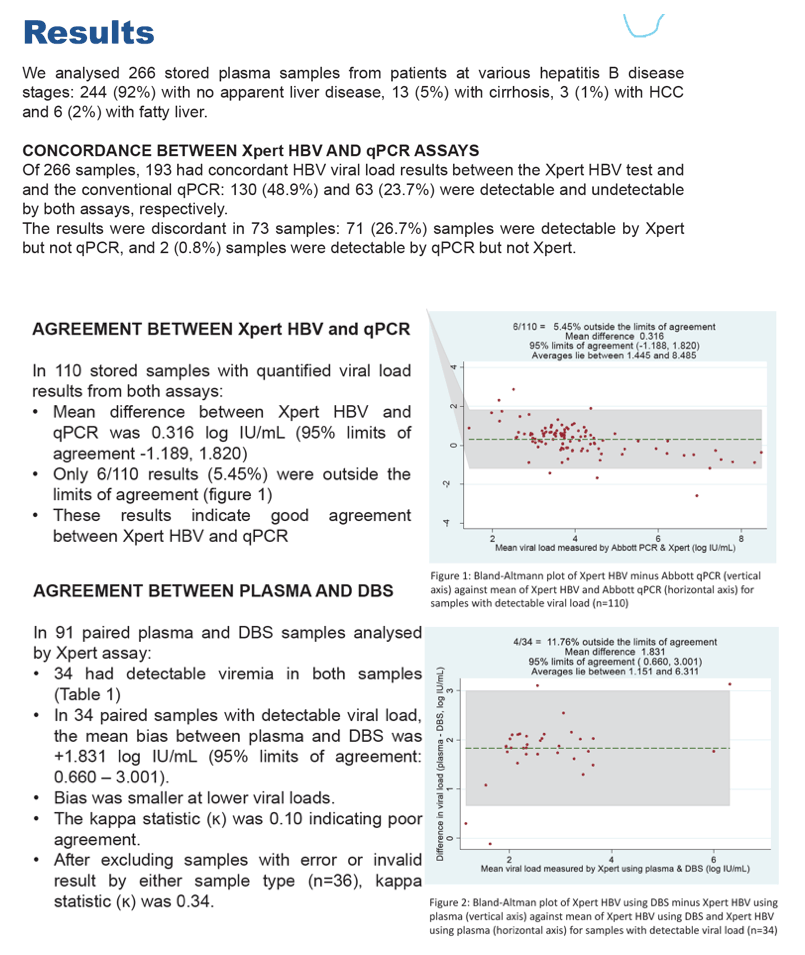
Results: We analysed 266 stored plasma samples from patients at various hepatitis B disease stages. Of these, HBV viral load results were concordant between the Xpert and the conventional qPCR: 130 (48.9%) and 63 (23.7%) were detectable and undetectable by both assays, respectively. The results were discordant in 71 (26.7%) samples that were detectable by Xpert but not qPCR, and 2 (0.8%) samples that were detectable by qPCR but not Xpert. In 110 samples with quantified viral load results from both assays, the mean difference between the two assays was 0.316 log IU/ml (95% limits of agreement - 1.189, 1.820) with only 6/110 results (5.45%) being outside the limits of agreement (figure 1), indicating good agreement.
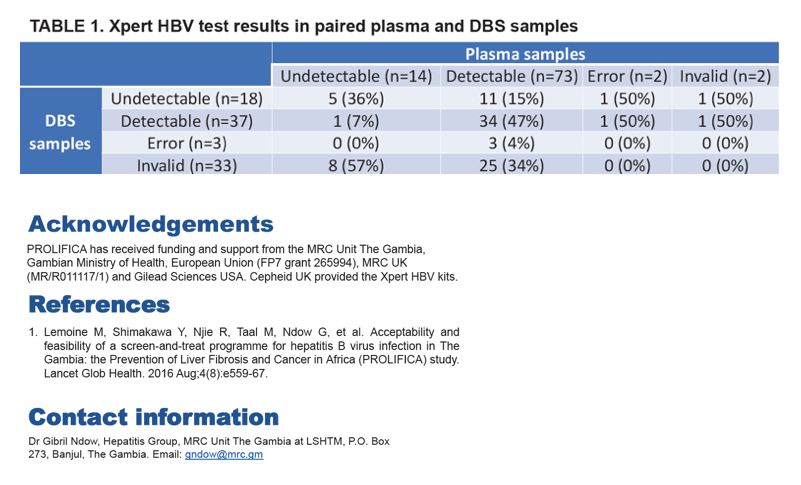
From 91 paired plasma and DBS samples analysed by Xpert assay, the mean bias between plasma and DBS was statistically significant at+1.831 log IU/ml (95% limits of agreement: 0.660-3.001). Bias was smaller at lower viral loads. The kappa statistic was 0.10 indicating very poor agreement between results. After excluding samples with error or invalid result by either sample type (n = 40), kappa statistic was 0.34 indicating a poor agreement.
Conclusion: The Xpert HBV DNA test provides a reliable alternative for detecting and quantifying HBV DNAviral load using stored plasma samples. However, the assay is not reliable for detecting viremia from DBS samples.

|
| |
|
 |
 |
|
|