 |
 |
 |
| |
Is it useful to add peg-IFN in poor responder patients to bulevirtide 2 mg? Results from the French multicenter early access program
|
| |
| |
PegIFNα May Rescue Bulevirtide Monotherapy Failure With HBV/HDV
AASLD 2023, The Liver Meeting, November 10-14, 2023, Boston
https://www.aasld.org/the-liver-meeting

Mark Mascolini
Adding weekly PegIFNα to failing 2-mg daily bulevirtide monotherapy in people with HBV/HDV coinfection induced good anti-HDV responses in a small study in France [1]. In another group getting 2 mg of bulevirtide with or without PegIFNα, boosting the dose from 2 to 10 mg did not control HDV well.
Bulevirtide, a novel inhibitor of HBV and HDV entry into liver cells, received conditional approval in Europe for treatment of chronic HBV/HDV coinfection in people with compensated cirrhosis. A phase 3 trial randomized coinfected people to 2 or 10 mg of subcutaneous bulevirtide daily or to a 48-week delay in therapy [2]. After 48 weeks, 45% in the 2-mg group, 48% in the 10-mg group, and 2% in the delayed-therapy control group attained the primary endpoint-a 2-log10 (100-fold)) drop in HDV load or undetectable HDV RNA (P < 0.001 for each bulevirtide arm vs control).
To learn more about the 50% of people who had an inadequate response to bulevirtide, researchers from across France focused on poor responders in the French bulevirtide early-access program. They had to be at least 18 years old with compensated cirrhosis or chronic hepatitis with at least moderate fibrosis (worse than F2) and high ALT liver enzyme readings. They all took bulevirtide at a dose of 2 mg subcutaneously daily for at least 3 months, with or without PegIFNα. The investigators retrospectively analyzed outcomes of people who had less than a 2-log10 (100-fold) drop in HDV RNA during treatment. They divided people into two groups: (1) those who switched from 2 to 10 mg of bulevirtide daily, and (2) those who maintained the 2-mg dose but added PegIFNα. The investigators defined virologic response as more than a 2-log fall in HDV load or an undetectable HDV load after at least 3 months of starting the new strategy.
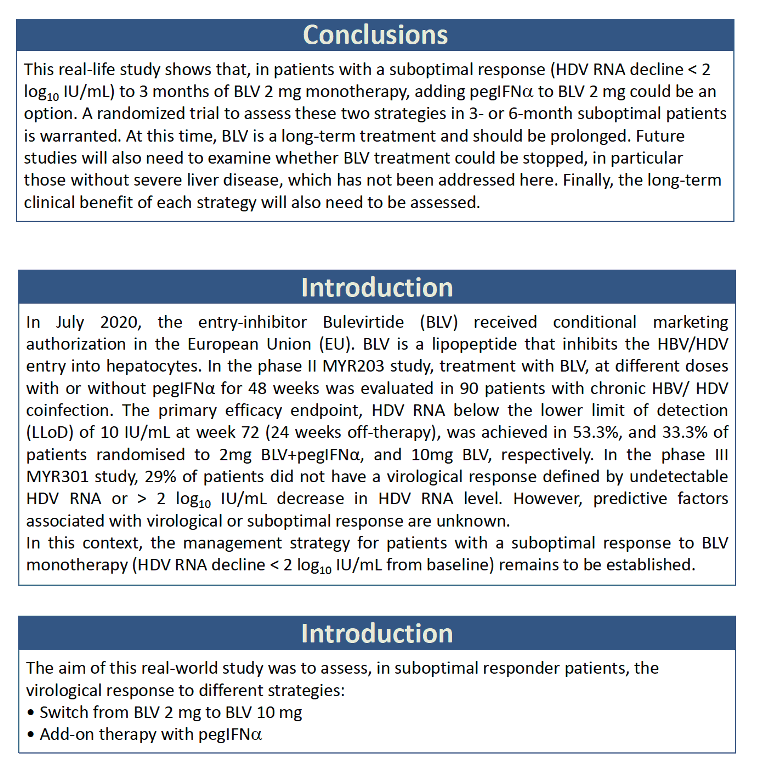
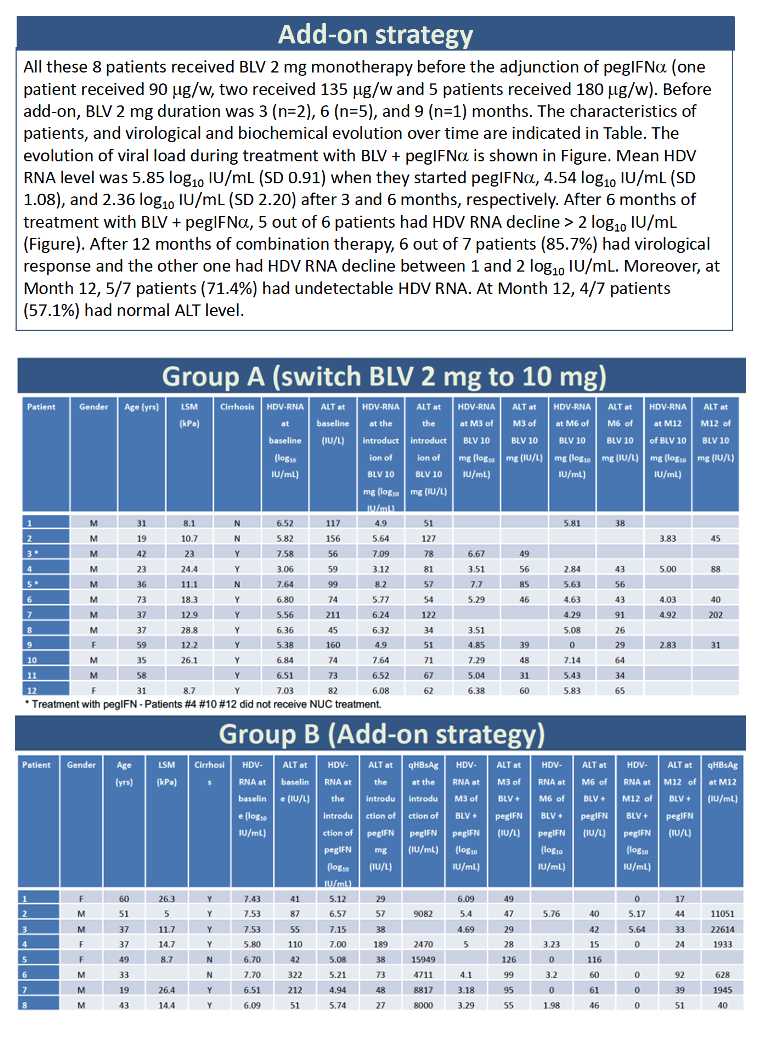
Twelve people made up the switch group, trading 2 mg of bulevirtide for 10 mg, with or without PegIFNα in either group. Before the switch, 1 of the 12 had taken 2 mg of bulevirtide for 4 months, 3 for 6 months, 1 for 7 months, 1 for 8 months, 5 for 9 months, and 1 for 12 months. HDV RNA averaged 6.03 log10 (over 1 million IU/mL) when they upped their dose from 2 to 10 mg daily, 5.58 log10 at 3 months after the dose increase, and 4.67 log10 at 6 months after the change. Only 1 of 9 people had more than a 2-log drop in HDV load at 3 months at the 10-mg dose, and only 2 of 10 at 6 months. Nobody in this dose-increase group reached an undetectable HDV load.
Among the 8 people who stayed with 2 mg of bulevirtide daily and added PegIFNα, 1 person added 90 ug weekly, 2 added 135 ug weekly, and 5 added 180 ug weekly. Two people had taken 2 mg of bulevirtide daily for 3 months, 5 for 6 months, and 1 for 9 months HDV RNA level averaged 5.85 log10 (about 631,000 IU/mL) when they started PegIFNα, 4.54 log10 after 3 months, and 2.36 log10 after 6 months. At the 6-month point, 5 of 6 people had pushed their HDV load down more than 2 log10. After taking PegIFNα with 2 mg of bulevirtide for 12 months, 6 of 7 people had a study-defined virologic response, and the remaining person had a 1- to 2-log10 fall in HDV load. After 12 months of combination therapy, 5 of 7 people had undetectable HDV RNA and 4 of 7 had a normal ALT.
On the basis of these add-on strategy results, the researchers proposed that starting PegIFNα in a person not responding to 2 mg of bulevirtide for 3 months "could be an option." They called for a randomized trial to assess the two strategies appraised here and suggested that future studies address whether bulevirtide can be stopped, especially in people without severe liver disease.
References
1. De Leadingham V, Hilleret MN, Metivier S, et al. Is it useful to add peg-IFN in poor responder patients to bulevirtide 2 mg? Results from the French multicenter early access program. AASLD 2023, The Liver Meeting, November 10-14, 2023, Boston.br clear="all" />
2. Wedemeyer H, Aleman S, Brunetto MR, et al. A phase 3, randomized trial of bulevirtide in chronic hepatitis D. N Engl J Med. 2023;389:22-32. doi: 10.1056/NEJMoa2213429. https://www.nejm.org/doi/10.1056/NEJMoa2213429

|
| |
|
 |
 |
|
|