Mother-to-Infant Transmission of Hepatitis C Virus
The full version of this article is available in PDF format.
Latifa T. F. Yeung,1 Susan M. King,2 And Eve A. Roberts 1
Hepatology Vol. 34, No. 2, August 2001
The rate of mother-to-infant HCV transmission is critical to predicting the burden of HCV infection in future generations. Mother-to-infant transmission of HCV may be intrauterine, intrapartum, or postnatal. The factors that determine whether or not an infant actually becomes infected need to be identified. If they can be manipulated, they represent areas for intervention to minimize mother-to-infant transmission. This review summarizes critically the current world-wide literature focusing on the rate of mother-to-infant transmission of HCV. Improved polymerase chain reaction (PCR) methods for detection of HCV RNA and larger studies will further refine the estimate of the incidence of mother-to-infant hepa-titis C.
An estimated 170 million people worldwide are chronically infected with HCV. If 35% of these are women of child-bearing age, given an annual fertility rate of 2%, then conservative estimates suggest that 10,000 to 60,000 newborn babies will be infected with HCV each year.
A review of published studies of mother-to-infant transmission of HCV was performed from January 1990 to December 2000. Articles were identified by computerized literature searches of MEDLINE and EMBASE, using combinations of key and free text terms (hepatitis C, mother-to-infant disease transmission, maternal-fetal exchange, placenta, mother-to-child, mother-to-infant, infant, pregnant, pregnancy). Bibliographies from review articles were manually cross-checked to identify additional references. No language restrictions were applied.
For this review, mother-to-infant transmission of HCV was defined as persistence of serum anti-HCV antibodies in the infant beyond at least 12 months of age or detection of serum HCV RNA on at least 1 occasion. Cord blood HCV RNA results were disregarded because of the possibility of contamination with maternal blood. Only original articles with at least 10 cases of anti-HCV–positive mothers were included. Studies using first-generation enzyme-linked immunosorbent assay (ELISA) or recombinant immunoblot assay (RIBA) tech-niques without confirmatory PCR testing were excluded.
Spontaneous clearance of mother-to-infant HCV infection (sometimes interpreted as transient viremia) was defined as seroreversion of serum HCV RNA (detected on at least one occasion and then subsequently found to be HCV-RNA nega-tive). This may be accompanied by elevated aminotransferases (ALT) during HCV-RNA positivity and subsequent loss of serum antibody-HCV.
Seventy-seven studies were included, published between 1992 and 2000. The number of anti-HCV–positive mother-infant pairs ranged from 10 to 1,338 per study. The articles in this review reported a total of 363 cases of mother-to-infant transmission. The majority of studies originated from Italy and Japan (Table 1). One Irish series focused on women who had received HCV-contaminated anti-D immunoglobulin. Almost all studies were prospective cohorts in design.
The quality of the studies was largely influenced by the number of mother-infant pairs studied (sample size), the definition of mother-to-infant transmission, the frequency and duration of infant follow-up, and the method of virologic test-ing. All studies were of cohort design (grade "C" evidence by Guyatt et al.16 guidelines).
Prevalence of Hepatitis C in Pregnant Women
The prevalence of antibody-HCV–positive women among all pregnant women varied widely across these studies (0.6%-95.4%), reflecting the heterogeneity of the populations studied. For example, the 3 studies with highest prevalence (70.1%-95.4%) were limited to intravenous drug users. Of anti-HCV–positive women, 26.8% to 94.4% tested positive for HCV RNA. For inclusion into this review, mother-to-infant transmission of HCV was defined as persistence of anti-HCV in the infant beyond at least 12 months of age or detection of serum HCV RNA on at least 1 occasion before 18 months of age. The majority of studies demanded at least one positive HCV RNA result. Some studies required more rigorous definitions of mother-to-infant transmission such as detection of antibody-HCV in the infant beyond 18 months of age, detection of HCV RNA after 6 months of age, detection of HCV RNA on at least 2 occasions, elevated serum aminotransferases, or matching genotype between mother and child.
Rate Of Mother-To-Infant Transmission
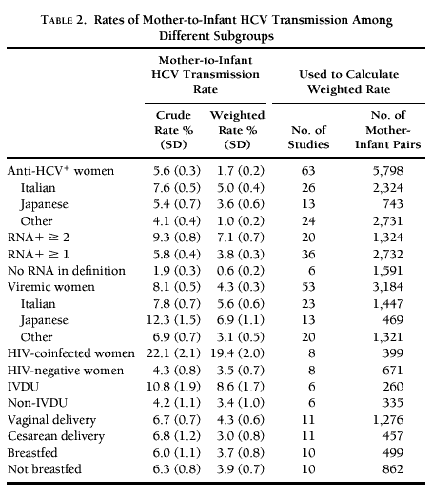
See Table 2below.
The reported rate of mother-to-infant HCV transmission ranged from 0% to 35.3% among children born to anti-HCV–positive women.
The definition of mother-to-infant transmission differed among studies. When all studies were grouped according to the rigorousness of definition of mother-to-infant transmis-sion (defined as two or more positive RNA, one or more positive RNA, or no positive RNA requirement), the weighted rates of mother-to-infant transmission among anti-HCV–positive women were 7.1% for studies requiring 2 or more positive RNA tests, 3.9% for studies requiring 1 or more positive RNA tests, and 0.6% for those with no requirement for a positive RNA test.
Human Immunodeficiency Virus Coinfection
The rate of mother-to-infant HCV transmission appears increased among women coinfected with human immunodeficiency virus (HIV) compared with women without HIV infection. Based on 8 studies that included data in both HIV-positive and HIV-negative women, the crude rate of mother-to-infant transmission was 22.1% and 4.3%, respectively. The weighted rate of mother-to-infant transmission was 19.4% and 3.5%, respectively.
Intravenous Drug Use
Patients reporting intravenous drug use (IVDU) have a high prevalence of chronic hepatitis C.29 Studies of mother-to-infant transmission among intravenous drug users and nonusers suggest that IVDU correlates with an increased risk of mother-to-infant HCV transmission. In 6 studies that included data on women with IVDU and without IVDU, the crude rate of mother-to-infant transmission among children born to anti-HCV–positive women with IVDU was 10.8%. The weighted rate was 8.6%. The corresponding crude and weighted mother-to-infant transmission rates for women who did not report a history of IVDU was 4.2% and 3.4%, respectively.
Maternal Viral Titer
A correlation exists between higher maternal titers of HCV RNA and the probability of mother-to-infant transmission. Although mother-to-infant transmission occurred across a wide range of maternal viral titers, in 9 studies statistically higher maternal viral titers corresponded to a greater tendency for mother-to-infant transmission; in 9 studies there was no difference. Most studies re-ported mother-to-infant transmission at viral titers beyond the range of 10 5 to 10 6 copies/mL. In one study higher levels of maternal serum HCV RNA also tended to correlate with higher colostrum levels of HCV RNA.47 The timing at which serum was collected for determination of maternal viral titer was reported with variable precision. Although most studies described blood collection at or near delivery, others ranged from the third trimester to after delivery. In 5 studies, including several in which maternal titer of HCV RNA was reported as having no bearing on rate of transmission, the timing of serum collection was unclear. (Commentary: these conflicting results may highlight a flaw with this study).
Mode Of Delivery
The mode of delivery was evaluated as a risk factor for mother-to-infant transmission in 11 studies. Between infants delivered vaginally versus by Cesarean section, overall rates of mother-to-infant transmission were similar. For vaginal delivery and Cesarean section respectively, the weighted rate of mother-to-infant transmission was 4.3% and 3.0%, respectively. Although Cesarean section was not further classified as elective or emergency, one study suggested an increased likelihood of mother-to-infant transmission with longer duration of membrane rupture. (Commentary: this also may highlight a study flaw as a number of studies do suggest that C-section may reduce transmission. These potential flaws also highlight the need to conduct more & better research in HCV).
Breast Feeding
The role of breast feeding was evaluated as a risk factor for mother-to-infant transmission of HCV in 10 studies. Only one of these studies defined the extent (duration and exclusivity) of breast feeding.24 Overall rates of mother-to-infant transmission between breast-fed and non–breast-fed infants were similar. The weighted rate of mother-to-infant transmission was 3.7% and 3.9% for breast-fed and non-breast-fed infants, respectively. Although some investigators have detected HCV RNA in breast milk, no definite case of mother-to-infant transmission of HCV via breast milk has been reported. (Commentary: again, I feel this may highlight a flaw in this study. The author says just above that only one study defined the extent of breastfeeding. This is an important question to try to answer but without rigorous studies I have to conclude at least for now that breast feeding does present a potential risk for HCV transmission and pregnant women should be so advised. Without realizing the limitations of these studies, individuals may underestimate the risks of breast feeding and report that there is no risk).
Genotype
Viral genotype was reported infrequently in 32 studies. In most cases, only the genotypes of vertically infected babies were noted. No conclusion could be drawn as to the effect of genotype on rate of mother-to-infant transmission.
Outcome Of Infants
Inconsistent follow-up among studies resulted in only sporadic description of clinical outcome for infected infants. No symptoms of liver disease were reported in the majority of case series; this may be an underestimate because many stud-ies focused on the rate of transmission rather than the long-term outcome. However, in one Egyptian study of 20 HCV-RNA– positive infants, 4 died of severe liver disease by 6 months of age. The remaining 16 infants were chronically sick with liver disease. Only 9 of these 16 infants later became asymptomatic despite remaining chronically infected. It is uncertain why these Egyptian infants were much sicker than other affected infants of HCV-infected women. Among studies describing mother-to-infant transmission rates, liver histology was described in only 17 infants. The reported age at time of liver biopsy ranged from 9 months to 5.5 years. The majority of liver biopsies revealed changes consistent with chronic hepatitis. Fibrosis on liver biopsy was described in 3 cases. One infant was successfully treated with interferon alfa.25 Three of the 17 infants died ;1 of these was coinfected with HIV 27 whereas another received 6 months of parenteral nutrition.
Spontaneous loss of serum HCV RNA, interpreted as either clearance of mother-to-infant HCV infection or transient viremia, was suggested in 59 cases. In these infants, serum HCV RNA was detected on at least one occasion and then the infant was subsequently found to be HCV-RNA negative. Many of these infants subsequently cleared serum anti-HCV. Six of these infants had elevated aminotransferases during the period of serum HCV-RNA positivity.
Passive transfer of maternal antibody accounted for a majority of infants born to women with hepatitis C. The gradual loss of serum anti-HCV in infants occurred by 18 months of age in the vast majority of cases, and many lost anti-HCV earlier, by 12 months of age. Of the 45 studies that described time to clearance of anti-HCV, there were only 3 outlying cases described, in whom clearance of anti-HCV occurred between 20 and 24 months of age.
Discussion
Several factors have been proposed as determinants in mother-to-infant HCV transmission, including maternal coinfection with HIV, IVDU, maternal viral titer, mode of delivery, breast feeding, and viral characteristics (e.g., genotype). In this summary, women coinfected with HIV or those who re-port a history of IVDU tended to have a higher rate of mother-to- infant transmission compared with those without such co-factors. By contrast, mode of delivery and prevalence of breast feeding did not significantly influence rates of mother-to-in-fant transmission. Suggested viral factors such as genotype and viral titer were not consistently measured across studies; their roles as significant risk factors in mother-to-infant transmission remain to be conclusively shown.
Furthermore, the suspected risk factors for mother-to-infant transmission deserve clarification. Some patients may not report a history of IVDU. Alternatively, some physicians may not probe carefully enough for a history of IVDU. Rather than mode of delivery, the extent of maternal-fetal blood exchange at time of delivery may be the major determinant of transmission as suggested by the duration of membrane rupture. Similarly, the definition of breast feeding remains ambiguous: a spectrum exists between being exclusively and never fed breast milk. Although maternal viral titer may serve as a useful risk factor, the timing of measurement for each woman relative to delivery should be specified. For maternal viral titer to become adopted as a prognostic factor in mother-to-infant HCV transmission, its timing will require standardization.
Thus far, the outcome of most infants with
chronic hepatitis C acquired through mother-to-infant transmission appears mild.
Most such children were asymptomatic. However, in the few infants who underwent
liver biopsy, most had evidence of chronic hepatitis. The progression of liver
disease for these children remains to be assessed. Fibrosis has been documented,
even in asymptomatic infants.
