Peginterferon
alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin
for initial treatment of chronic hepatitis C: a randomised trial
Michael P Manns, John G McHutchison, Stuart C
Gordon, Vinod K Rustgi, Mitchell Shiffman, Robert Reindollar, Zachary
D Goodman, Kenneth Koury, Mei-Hsiu Ling, Janice K Albrecht, and the
International Hepatitis Interventional Therapy Group*
THE LANCET Vol 358
September 22, 2001
ABSTRACT:
1530
patients with chronic hepatitis C were assigned interferon alfa-2b (3 MU
subcutaneously three times per week) plus ribavirin 10001200 mg/day orally,
peginterferon alfa-2b 1·5 g/kg each week plus 800 mg/day ribavirin, or
peginterferon alfa-2b 1·5 g/kg per week for 4 weeks then 0·5 g/kg per week
plus ribavirin 10001200 mg/day for 48 weeks. The primary endpoint was
the SVR rate (undetectable
hepatitis C virus [HCV] RNA in serum at 24-week follow-up). Analyses were based
on patients who received at least one dose of study medication.
The SVR rate was
significantly higher (p=0·01 for both comparisons) in the higher-dose
peginterferon group (274/511 [54%]) than in the lower-dose peginterferon
(244/514 [47%]) or interferon (235/505 [47%]) groups. Among patients with HCV
genotype 1 infection, the corresponding SVR rates were 42% (145/348), 34%
(118/349), and 33% (114/343). The rate for patients with genotype 2 and 3
infections was about 80% for all treatment groups. Secondary analyses identified
bodyweight as an important predictor of SVR, prompting comparison of the
interferon regimens after adjusting ribavirin for bodyweight (mg/kg).
Side-effect profiles were similar between the treatment groups.
REPORT
Histological response
was assessed for inflammation and fibrosis by the Knodell histological activity
index. 16,17 The inflammation score was obtained by combining scores for the
first three components of the Knodell index: portal, periportal, and lobular
inflammation (range 018, with higher scores indicating more severe
abnormalities); improvement was defined as a decrease in the inflammation score
of at least 2 units. The Knodell fibrosis scores are 0 (no fibrosis), 1 (portal
fibrosis), 3 (bridging fibrosis), and 4 (cirrhosis). 16,17 An improvement in
fibrosis was defined as a decrease of 1 or more from the pretreatment to
post-treatment Knodell fibrosis score, and a worsening of fibrosis was defined
as an increase in this score of 1 unit or more.
RESULTS
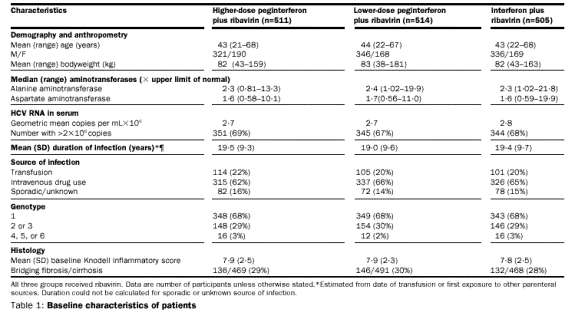
Patients
characteristics Enrolment began in March, 1998, and the trial was completed in
October, 2000. A total of 2316 patients were screened, and 1530 were enrolled
and treated (figure 1). All efficacy and safety analyses were based on the 1530
patients who received at least one dose of medication.
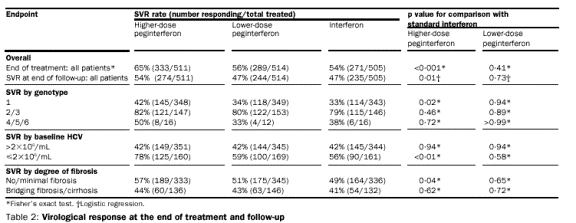
Responses
The SVR rate was
significantly higher for the group receiving peginterferon alfa-2b at the higher
dose of 1·5 g/kg per week than for the other two treatment groups (54 vs 47%,
table 2). The benefit of the higher-dose regimen was most apparent for patients
with genotype 1 infection, the subgroup that is most common and difficult to
treat. The biochemical (alanine aminotransferase) response rates at the end of
treatment were similar in the three treatment groups: 65%, 63%, and 69% for the
higher-dose peginterferon, lower-dose peginterferon, and interferon groups,
respectively. The sustained response rates in terms of alanine aminotransferase
were higher (54%) among the group assigned the higher dose of peginterferon than
in those assigned the lower dose of peginterferon (48%) or interferon alfa-2b
(47%). Nearly all patients who had SVRs also had normal alanine aminotransferase
values at the end of follow-up: 97% of the higher-dose peginterferon group, 92%
of the lower-dose peginterferon group, and 97% of the group. The
proportion of patients who cleared virus but who relapsed by the end of
follow-up was low in all treatment groups (18%, 16%, and 14%, respectively).
As previously shown for therapy with interferon alfa-2b plus ribavirin, late
viral clearance at week 12 or 24 and subsequent SVR were also observed in
patients receiving peginterferon alfa-2b plus ribavirin. In the group assigned
the higher dose of peginterferon alfa-2b, 75%
of patients who were HCV RNA negative for the first time at week 12 achieved
SVRs, and 32% of patients who lost HCV RNA for the first time at week 24 of
therapy achieved SVRs. Previous studies with interferon alfa-2b plus
ribavirin have shown that, in general, if patients do not respond by

treatment week 24, an SVR will
not be achieved; a similar pattern is observed with peginterferon alfa-2b plus
ribavirin. Among the patients who had no detectable viral RNA for the first time
more than 24 weeks after the start of treatment, few patients achieved SVRs
(four patients treated with higher-dose peginterferon alfa-2b; none treated with
lower-dose peginterferon alfa-2b; and one treated with interferon alfa-2b).
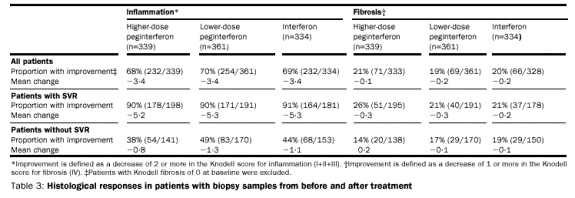
Biopsy samples from before and after treatment were available in 1034 (68%)
patients. Histological inflammation improved in all three treatment groups
(table 3). Histological improvement was also greatest in sustained responders;
90% of this subgroup had histological improvement, irrespective of the treatment
regimen they received. Among non-responders, 44% (205/464) also had some
evidence of histological. Improvement in fibrosis was observed in about 20% of
patients in each treatment group.
Variables associated with SVR
(Comments from Jules Levin: the question of whether
or not ribavirin dosing should be weight based has been controversial. In this
study, the authors take a retrospective look at the concentration of ribavirin
based on the weight of the patient. And they find the higher the concentration
of ribavirin the better the response to treatment. But they also find that
baseline weight (lighter) is associated with SVR. To me, this makes it difficult
to conclude which may be the reason for better response lighter weight or
receiving higher concentrations of ribavirin or both. The authors make a case
below that concentration of ribavirin was a significant factor upon further
statistical analysis. Nonetheless, these studies are retrospective and the FDA
did not approve ribavirin weight based dosing when PegIntron/RBV was recently
approved. The European regulatory authorities did, however, approve ribavirin
weight based dosing. Schering is
conducting a large prospective study to establish the usefulness of RBV weight
based dosing).
To examine the influence
of potentially important prognostic factors on SVR, factors known to affect
response (HCV genotype, baseline viral load, cirrhosis, age, sex, baseline
weight) were first examined individually by univariate logistic regression
analysis on each factor for all treatment groups combined. HCV genotype (other
than genotype 1), the baseline viral load (log; lower viral load), baseline weight (lighter), and age (younger) were clearly associated
with SVR (p<0·0001), as were sex (p=0·01) and, to a lesser extent, the
absence of cirrhosis (p=0·07). Since only 57% of patients had cirrhosis at
baseline, this analysis may not have been sensitive enough to detect a clear
relation between cirrhosis and SVR. However, the absence of bridging
fibrosis/cirrhosis was
significantly associated with
SVR (p=0·001). To assess the independence of these factors, a backward
elimination procedure was then used. All variables identified by univariate
analyses were retained in the final multivariate
model, except sex.
Specifically, although weight was retained (p=0·03), sex was no longer
significant when weight was taken into account (p=0·35). Doses of peginterferon
alfa-2b are given according to the patients weight, because of the previous
observation with interferon alfa-2b that among patients receiving 3 MU three
times per week, lighter patients have higher SVR rates than heavier patients. In
this study, logistic regression analysis showed that baseline weight was an
important predictor of SVR. Because a single dose of ribavirin (800 mg) was used
with the higher dose of peginterferon alfa-2b, the effect of the ribavirin dose
adjusted for bodyweight (mg/kg) was explored. Logistic regression analyses were
used to characterise further the relation between SVR and the doses of both
peginterferon alfa-2b and ribavirin. The two doses of peginterferon alfa-2b were
treated as a categorical variable, and the dose of ribavirin as a continuous
variable, with each patients ribavirin dose expressed as mg/kg. The results
indicate that the doses of both drugs are important and significantly predict
SVR (odds ratio 1·7, p=0·002 for higher-dose vs lower-dose peginterferon
alfa-2b, and slope 0·07, p=0·015 for ribavirin). Thus, the likelihood of SVR
increases as ribavirin dose increases. Also, when the dose of ribavirin is
controlled on a mg/kg basis, the estimated effect of the higher dose of
peginterferon alfa-2b compared
with the lower dose was larger
(odds ratio 1·7) than the estimate obtained from the primary (as-randomised)
analysis (1·3). To account for possible interaction between the two drugs, a
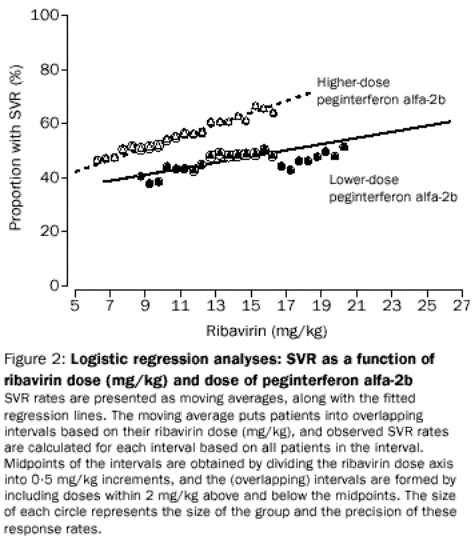
term for the product of the two doses was included in the model. Fitted
regression lines are shown in figure 2, which also shows that the observed
response rates (moving average) generally increase as ribavirin dose increases
up to about 13 mg/kg. The observed response rates were almost flat between 13
and 15 mg/kg; although the number of patients was small, the observed response
rates did not increase after 15 mg/kg. From these results, the ribavirin dose
range that allowed for adequate interpretation of both safety and efficacy data
was 1115mg/kg. Thus, for an average 75 kg man, doses of
10·6 mg/kg, 13·2 mg/kg, and
about 15 mg/kg represent daily doses of 800 mg, 1000 mg, and 1200 mg ribavirin,
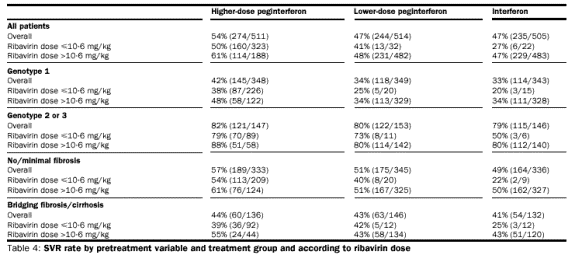
respectively. SVR rates are presented in table 4 according to weight-based
ribavirin dose (10·6 mg/kg or less, and more than 10·6 mg/kg) according to
treatment group and stratification factors. The SVR rate was higher in all
groups when the dose of ribavirin was greater than 10·6 mg/kg bodyweight, which
is the lower end of the optimum dose range. For genotype 1 patients who received
more than 10·6 mg/kg ribavirin the SVR rate was in the higher-dose
peginterferon group than in the other two groups.
Influence of other prognostic
or potentially confounding factors
Both the fitted
regression lines and the observed response rates show that the likelihood of an
SVR can be increased in patients treated with the higher dose of peginterferon
alfa-2b by increasing the ribavirin dose into the range obtained by the
10001200 mg dosing schedule in the other two treatment groups. However,
because these analyses were not based on direct comparisons of the as randomised
treatment groups, we had to assess the potential for imbalances in important
prognostic factors to bias the assessment of treatment effects. An obvious
potential confounding factor is sex, since women tend to weigh less than men and
therefore receive higher doses of ribavirin per unit of bodyweight. To confirm
the importance of treatment effects after the potentially confounding factors
were taken into account, multivariate logistic regression analyses were done.
Each analysis included treatment effects, interferon alfa-2b dose (1·5 g/kg per
week vs others), and ribavirin dose (mg/kg), as well as HCV genotype and (log)
baseline viral load. Other potential confounding factors identified above (ie,
sex, age, bridging fibrosis/cirrhosis) were then added one at a time. In each
case, both treatment effects (peginterferon alfa-2b and ribavirin) remained
significant after all other factors were taken into account, and both genotype
and baseline viral load were independent predictors of response (p<0·0001).
When the dose of ribavirin was expressed as mg/kg, sex was not significant (p=0·54).
Similarly, weight was no longer significant (p=0·30) after control for
ribavirin dose (mg/kg). Age and the presence of bridging fibrosis/cirrhosis
remained independent predictors of response (p<0·01).
Safety evaluation
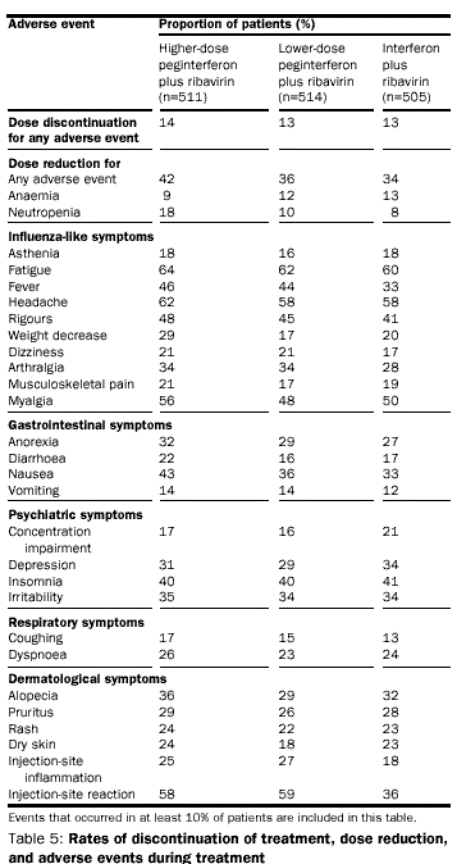
The
side-effect profiles of peginterferon alfa-2b plus ribavirin and interferon
alfa-2b plus ribavirin were similar; there were no new or unique adverse events
(table 5). The rate of several influenza-like symptoms was higher in the
higher-dose peginterferon group than in the interferon alfa-2b group, probably
because a higher dose of interferon alfa-2b is administered via the
peginterferon formulation. As previously reported with peginterferon alfa-2b
monotherapy, there was a substantial increase in injection-site reactions
compared with interferon alfa-2b.
In
all cases, the characteristics of the reaction were similar for both interferon
alfa-2b and peginterferon alfa-2b. The typical event was generally mild, not
treatment-limiting, and characterised by localised erythema. Anaemia is a well-recognised
effect of ribavirin, and the pattern that has been previously observed was seen
in this study. The optimum treatment regimen of peginterferon alfa-2b 1·5 g/kg
plus ribavirin did not result in a greater fall in haemoglobin; the mean
decreases at weeks 48 were the same (25 g/L), for both that group and the
standard interferon group. A decrease in haemoglobin to less than 100 g/L, the
protocol requirement for dose modification, occurred in 9% of patients receiving
the higher-dose peginterferon alfa-2b plus ribavirin regimen and 13% of patients
receiving interferon alfa-2b. Discontinuation for anaemia was rare.
The frequency of dose reduction for neutropenia
according to the protocol was 18% for the higher-dose peginterferon alfa-2b plus
ribavirin regimen compared with 8% for interferon alfa-2b plus ribavirin. However, or 1% less of patients discontinued
treatment for neutropenia, which suggests that the dose-modification schedule
included in the protocol provided good protection for the patient. The effect of
combination therapy on platelet counts reflects a balance between the effects of
interferon alfa-2b and ribavirin; the former reduces platelet counts, while the
latter is associated with a reactive thrombocytosis. 3% of patients in the
higher-dose peginterferon group and 1% in the interferon alfa-2b group had a
platelet decrease that reached the protocol-defined criterion for dose
reduction. No patient discontinued
therapy owing to thrombocytopenia. No
significant increase in alanine aminotransferase (greater than five times
baseline) was observed in any patient during the study. Rises in alanine
aminotransferase more than two times baseline were infrequent, occurring in 3%,
2%, and 1% of patients receiving ribavirin in combination with higher-dose
peginterferon, lower-dose peginterferon, and standard interferon alfa-2b,
respectively. The safety profile for patients receiving a dose of more than 10·6
mg/kg ribavirin with either interferon formulation was similar. Only a few
adverse events were more frequent (>10% difference) in this group (asthenia,
weight loss, nausea, and alopecia).
Among patients receiving more than 10·6 mg/kg
ribavirin, dose modification was more frequent with alfa-2b than with interferon
alfa-2b (49% vs 34%),
with most of the difference due to neutropenia in the peginterferon alfa-2b
group. By contrast, the discontinuation rate in the two treatment groups was
similar, 14% vs 13%, respectively. A decrease in haemoglobin to less than 100
g/L or the need for dose reduction owing to anaemia were neither more frequent
nor of greater severity in patients who received the higher dose of
peginterferon alfa-2b and ribavirin more than 10·6 mg/kg.
Discussion
Late viral clearance was
infrequently associated with eventual SVR in this study. Only one of 403
patients who received either dose of peginterferon alfa-2b plus ribavirin and
who had detectable HCV RNA at week 24 of therapy showed an SVR, which is
consistent with previous studies in patients treated with standard interferon
alfa-2b plus ribavirin. This finding supports the current recommendation to
consider stopping therapy at week 24 in genotype-1-infected patients with
persistent viraemia. However, such a strategy does not take into account a
report that hepatitis C patients treated with interferon-based regimens who do
not achieve eradication of HCV RNA from serum or normalisation of liver enzymes
may derive some short-term histological benefit.
20 Further prospective trials
are in progress to find out whether these potential antifibrotic effects of
interferon will be valuable in preventing disease and fibrosis progression. As
in previous studies, 5% of patients had persistent rises in alanine
aminotransferase during follow-up, but they had eradicated HCV RNA from serum
and were classified as sustained responders. 21 The reasons for these discordant
results remain unclear, but previous studies have also indicated that some of
these patients may have steatosis or other potential causes for the persistently
abnormal biochemical results. 14 The most effective therapy for the initial
treatment of suitable patients with chronic hepatitis C according to this study
is the combination of 1·5 g/kg per week of peginterferon alfa-2b with ribavirin.
Because of the improved response rate and more convenient once-weekly
administration schedule, this regimen will replace the current standard of
interferon alfa-2b plus ribavirin.
References
1 Alter MJ, Kruszon-Moran D, Nainan OV, et al. The
prevalence of hepatitis C virus infection in the United States, 1988 through
1994. N Engl J Med 1999; 341: 55662.
2 National Institutes of Health Consensus
Development Conference Panel Statement: Management of Hepatitis C. Hepatology
1997; 26 (suppl 1): 210.
3 Seeff LB, Buskell-Bales Z, Wright EC, et
al. Long-term mortality after transfusion associated non-A, non-B hepatitis. N
Engl J Med 1992; 327: 190611.
4 Tong MJ, el-Farra NS, Reikes AR, Co RL. Clinical
outcomes after transfusion-associated hepatitis C. N Engl J Med 1995; 332:
146366.
5 Darby SC, Ewart DW, Giangrande PL, et al.
Mortality from liver cancer and liver disease in haemophilic men and boys in UK
given blood products contaminated with hepatitis C. Lancet 1997; 350: 142531.
6 Detre KM, Belle SH, Lombardero M. Liver
transplantation for chronic viral hepatitis. Viral Hepatitis Rev 1996; 2:
21928.
7 McHutchison JG, Gordon SC, Schiff ER, et
al. Interferon alfa-2b
alone or in combination with ribavirin as initial
treatment for chronic hepatitis C. N Engl J Med 1998; 339: 148592.
8 Poynard T, Marcellin P, Lee SS, et al.
Randomised trial of interferon alfa-2b plus ribavirin for 48 weeks or for 24
weeks versus interferon alfa-2b plus placebo for 48 weeks for treatment of
chronic infection with hepatitis C virus. Lancet 1998; 352: 142632.
9 Glue P, Fang JW, Rouzier-Panis R, et al.
Pegylated interferon alfa-2b:
pharmacokinetics, phamacodynamics, safety and
preliminary efficacy data. Clin Pharmacol Ther 2000; 68: 55667.
10 Zeuzem S, Feinman SV, Rasenack J, et al.
Peginterferon alfa-2a in patients with chronic hepatitis C. N Engl J Med 2000;
343: 166672.
11 Lindsay K, Trepo C, Heintges T, et al. A
randomised, double-blind trial comparing pegylated interferon alpha-2b to
interferon alpha-2b as initial treatment for chronic hepatitis C. Hepatology
2001; 34: 395403.
12 Glue P, Rouzier-Panis R, Raffanel C, et
al. A dose-ranging study of pegylated interferon alfa-2b and ribavirin in
chronic hepatitis C. Hepatology 2000; 32: 64753.
13 Buti M, Olive G, Stalgis C, Esteban R,
Guardi J. Quantification of serum hepatitis C virus RNA with daily or standard
interferon doses plus ribavirin in nonresponder patients with chronic hepatitis
C. Dig Dis Sci 2000; 45: 68589.
14 Tong MJ, Blatt LM, McHutchison JG, Co RL,
Conrad A. Prediction of response during interferon alfa-2b in chronic hepatitis
C patients using viral and biochemical characteristics: a comparison. Hepatology
1997; 26: 164045.
15 Stuyver L, Rossau R, Wyseur A, et al.
Typing of hepatitis C virus isolates and characterization of new subtypes using
a line probe assay. J Gen Virol 1993; 74: 1093102.
16 Knodell RG, Ishak KG, Black WC, et al.
Formulation and application of a numerical scoring system for assessing
histological activity in asymptomatic chronic active hepatitis. Hepatology 1981;
1: 43135.
17 Goodman ZD, Ishak KG. Histopathology of
hepatitis C virus infection. Semin Liv Dis 1995; 15: 7081.
18 Carithers RL, Zeuzem S, Manns MP, et al.
Multicenter, randomized controlled trial comparing high dose daily induction
interferon plus ribavirin versus standard interferon alfa-2b plus ribavirin.
Hepatology 2000; 32: 317A (abstr).
19 He X-H, Shaw P-C, Tam S-C. Reducing the
immunogenicity and improving the in vivo activity of trichosanthin by
site-directed pegylation. Life Sci 1999; 65: 35568.
20 Shiffman ML, Hofmann CM, Contos MJ, et al.
A randomized, controlled trial of maintenance interferon therapy for patients
with chronic hepatitis C virus and persistent viremia. Gastroenterology 1999;
117: 1164172.
21 Blatt LM, Tong MJ, McHutchison JG, Russell
J, Schmid P, Conrad A. Discordance between serum alanine aminotransferase (ALT)
and virologic response to IFN-alpha2b in chronic hepatitis C patients with high
and low pretreatment serum hepatitis C virus RNA titers. J Interferon Cytokine
Res 1998; 18: 7580.