 |
 |
 |
| |
"Interferon alfa-2b plus Ribavirin for HCV-infected Patients with Normal ALT"
|
| |
| |
...response rates were comparable to patients with normal ALT. The trend towards superiority of IFN 5 MU leaves open question on optimal dosing. Combination therapy is safe- de novo ALT elevations are seldom problematic...
For background Ira Jacobson (Cornell Hospital, NYC) reported that persistently normal ALT is common with HCV. Histology (liver disease) is often mild and progression slow in the HCV mono-infected patient. In the HCV/HIV coinfected patient a number of studies show that HCV disease progression can be accelerated by HIV. In the mono-infected patient <5% to 20% have significant hepatic fibrosis. Interferon therapy has been associated with leading to elevated ALT 40% of the time in patients with normal ALT. At the 1997 NIH Consensus Conference treatment of patients with normal ALT was considered investigational. The next NIH Consensus Conference takes place this June 2002.
The aim of this study is to assess the efficacy and safety of 2 different doses of alfa-2b interferon combined with ribavirin in HCV+ patients with normal ALT. To qualify for this study, patients had to have a biopsy consistent with chronic hepatitis; normal ALT on 2 or more occasions at least 3 months apart; compensated liver disease. Patients were randomized to receive one of two regimens: group 1- IFN alf-2b 3Million Units 3 times per week + ribavirin 1000-1200 mg/day; or, group 2- interferon alfa-2b 5 MU 3 times per week + ribavirin 1000-1200 mg/day. Treatment was for 48 weeks, and if patients were HCV-RNA positive at week 24 they could stop therapy. There was a follow--up period of 24 weeks following therapy cessation to assess the 72 week SVR.
There were 28 patients in each group, 41% male; 69% genotype 1 in 3 MU group and 81% genotype 1 in 5 MU group. 24% genotype 2/3 in 3 MU group vs 11% in 5 MU group. Mean ALT was 35 in group 1 and 31 in group 2. Fibrosis stage 0-1: 18% in each group. Fibrosis stage 2-4 11% in group 1, 9% in group 2.
16 patients had stage 0. 20 patients had stage 1. 13 patients had stage 2. 6 patients had stage 3. 1 patient had stage 4. So, 7/28 (25%) patients with apparently normal ALT had stage 3/4.
RESULTS
The overall response rate was 32% for all patients , but 28% for the 3 MU group and 40% in the 5 MU group. Genotype 1 patients receiving 5 MU had 40% SVR vs 10% for patients receiving 3 MU. For genotype 2/3 patients over 80% receiving 3 MU had SVR and 70% receiving 5 MU had SVR.
3 patients relapsed after ending 48 weeks treatment. 4 patients had viral breakthrough on therapy. 32% of patients with stage 0,1 (n=36) had SVR and 30% with stage 2, 3, 4 had SVR. 40% of patients with <2 million HCV-RNA had SVR and 20% with >2 million had SVR.
|
|
 |
| |
Adverse Events
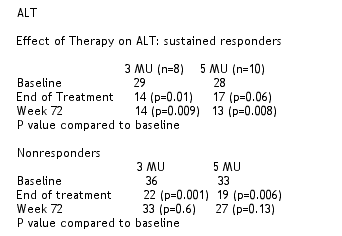
5 patients experienced ALT elevations. All were reportedly transient, one with transient 2.2 bilirubin. They were not more than 2x upper limit of normal. There were 2 serious adverse events: 1 suicidal ideation; confusion, anxiety. 9/56 patients (16%) discontinued due to an adverse event. Dose modifications were similar in both groups. There were no unexpected adverse events.
Jacobson concluded there was a trend towards a better response with IFN 5 MU in patients with genotype 1. ALT elevations were infrequent, mild, and transient. Response in stage 2-4 was similar to stage 0-1. ALT was reduced during treatment and in SR. AE, and serious AE was similar as in other studies. Response rates were comparable to patients with normal ALT. The trend towards superiority of IFN 5 MU leaves open question on optimal dosing. Combination therapy is safe- de novo ALT elevations are seldom problematic. Treatment should be individualized based on patient characteristics, including history. Therapy should not be ruled out based on ALT alone. This trial was conducted before pegylated IFN was available, so studies using peg IFN in patients with normal ALT are in progress.
|
|
| |
|
 |
 |
|
|