 |
 |
 |
| |
Review of Oral Presentations and Posters presented at DDW May 19-20
Written by Andrew Talal, MD, NY Hospital-Cornell Medical College, Dept of GI & Hepatology
|
| |
| |
Below is a summary of the relevant oral and poster presentations related to hepatitis C virus (HCV) and HIV/HCV coinfection that were presented at Digestive Disease Week in San Francisco on Sunday, May 19 and Monday, May 20. One of the most notable features of the DDW meeting this year is the paucity of abstracts related to HIV in the gastrointestinal tract and related to HIV/HCV coinfection. Additionally, many of the oral presentations were preliminary or interim results from studies that are currently in progress. The following are the abstracts that are of interest to the HIV-treating physician the majority of which are related to HCV monoinfection.
I. AASLD Topic Forum: HCV Treatment
The abstracts presented during this session primarily dealt with issues related to the treatment of HCV. The abstracts presented in the next section dealt with issues related to HCV treatment in special populations of patients. The presentations in the third section dealt with issues related to liver transplant.
1) Pegylated Interferon alfa-2b (PegIntron) Plus Ribavirin in Patients with Chronic Hepatitis C: A Trial in Prior Nonresponders to Interferon Monotherapy or Combination Therapy and in Combination Therapy Relapsers. Abstract #79
These authors presented the results of a trial that is designed to assess the efficacy of pegylated interferon and ribavirin in individuals who either did not respond (nonresponders) or relapsed after treatment for HCV. Since sixty percent of HCV-infected individuals do not respond to combination therapy, the goal of this study was to evaluate the sustained virologic response (SVR) using two different doses of pegylated interferon and ribavirin in: 1) prior nonresponders to IFN monotherapy, 2) prior nonresponders to combination therapy, and 3) individuals who initially had HCV RNA below assay detection who subsequently relapsed after stopping therapy (relapsers). Approximately forty percent of individuals who initially respond to combination interferon and ribavirin therapy subsequently relapse.
Subjects were randomized to receive: 1) peg-IFN 1.0 ug/kg plus RBV 1000-1200 mg per day (group 1) or 2) peg-IFN 1.5 ug/kg plus RBV 800 mg per day (group 2). Subjects were treated for a total of 48 weeks unless the Week 24 HCV RNA was still detectable. HCV RNA was measured by the Roche Amplicore assay at baseline, and at Weeks 24, 48, and 72.
Among the individuals who failed to achieve a response with combination therapy consisting of standard interferon and ribavirin, 10% of the genotype 1 and 25% of the genotype 2 infected individuals achieved an SVR. The response was much higher in those individuals who relapsed after a course of combination therapy. Approximately one quarter to one third of the individuals who not respond to interferon monotherapy will achieve an SVR to therapy with pegylated interferon and ribavirin. In addition, the response was higher in individuals with more severe scaring in the liver to the higher dose of pegylated interferon and ribavirin. As has been shown in other studies, the SVR in African Americans was less than it was in Caucasians (20% vs. 8%).
In conclusion, this study shows that individuals who did not respond or who relapsed after treatment with interferon monotherapy or combination therapy should be treated with pegylated interferon and ribavirin. Furthermore, the response did not differ significantly between the two doses of interferon that were used in this study. The study also showed that the response is higher in patients who relapse after treatment then it is in patients who do not respond at all to the initial therapy.
2) Pegylated Interferon Alfa-2b (PegIntron) and Ribavirin for Hepatitis C in Patients who were Nonresponders to Previous Therapy; Induction Therapy. Abstract #80
Studies in which HCV RNA (indicating the amount of hepatitis C in the blood) is measured frequently after starting anti-HCV medication have shown that the faster that the HCV RNA declines, the more likely an individual is to achieve a response to anti-HCV medication. However, studies of induction therapy, in which a higher dose of medication is given for a short period of time at the beginning of the study in an attempt to decrease the amount of virus as much as possible, have not shown a clear advantage. The primary aim of this study was to evaluate the SVR in response to induction therapy with Peg-Intron /RBV compared to fixed dose Peg-Intron/RBV in previous standard therapy nonresponders, IFN monotherapy nonresponders, and combination therapy relapsers.
A total of 680 individuals enrolled in the study, 473 who were prior combination therapy nonresponders, 108 IFN monotherapy nonresponders, and 99 combination therapy relapsers with compensated liver disease. Participants were randomized to either: 1) the induction dose group-Peg-Intron 1.5 mcg/kg/wk + RBV 1000-1200 mg per day for 12 weeks followed by Peg-Intron 1.0 mcg/kg/wk + RBV 800 mg per day for 36 weeks, or 2) the fixed dose group-Peg-Intron 1.0 mcg/kg/wk + RBV 800 mg. per day for a total of 48 weeks. HCV RNA was determined at Week 0, 24, and 48 after treatment initiation and 24 weeks after treatment cessation.
The presentation included data after 24 and 48 weeks of treatment. Overall, 48% of the individuals in this study responded and individual subgroup responses were: 25% of the prior nonresponders to standard combination therapy, 30-34% of interferon monotherapy nonresponders, and 55% of relapsers. Of the combination therapy nonresponders, induction therapy appeared to improve the 48 week response rate, as only 15% of those in the fixed dose group responded while 25% in the induction dose group responded. Among the combination therapy nonresponders with genotype 1, induction therapy increased the response from 21% to 29%. Induction therapy did not appear to substantially change the response among interferon monotherapy nonresponders and combination therapy relapsers. Of note, induction therapy increased the response among prior interferon monotherapy nonresponders with stage 3-4 from 33% to 45%, although only a small number of individuals were included in this subgroup.
Induction dosing with Peg-Intron/RBV, compared to fixed dose, appears to have the greatest benefit among combination therapy nonresponders, particularly those infected with genotype 1. We will anxiously await the 24-week follow-up data to determine if the trends that have been observed at 24 and 48 weeks will continue.
3) Prediction of Early Virologic Response to Treatment with 40kD Pegylated-Interferon Alfa 2a (Pegasys) / Ribavirin in Patients with Chronic Hepatitis C (Genotype 1). Abstract #81
Reliable predictors of sustained virologic response are desperately needed to avoid prolonged exposure to interferon among individuals with a limited likelihood of achieving a therapeutic response. The absence of HCV RNA in the blood 12 weeks after starting interferon can be used to predict those individuals who are likely to achieve an SVR. The absence of a significant decline in the viral load (> 0.8 log) within the first 24 hours is very predictive of nonresponse to interferon. The goal of this study was to evaluate whether a test dose of interferon administered within the first 24 hours was predictive of a response at week 12.
Chronically HCV-infected genotype 1 individuals were selected from a prospective randomized trial evaluating amantidine compared to placebo in combination with Peg-IFN alfa-2a (Pegasys) 180 mcg/wk and RBV 1000-1200 mg per day. Subjects were given a dose of standard IFN 9 MU at baseline and after 24 hours. Subjects were stratified based upon HCV RNA at 24 hours after the test dose. Subjects were then divided into two groups depending upon: 1) the amount of scaring in the liver and 2) the amount of decline of HCV RNA.
The data presented were an interim analysis of the Week 12 and 24 responses among a third of the total number of anticipated participants that the authors expect to enroll in the trial. There was a trend toward a lower viral load in those who cleared virus at Week 24 compared to those who did not (607,000 vs. 400,000; p=0.09). Furthermore, the decline in the first 24 hours was much greater in those who cleared virus by Week 24 compared to those who did not (1.17 +/- 0.07 among those without detectable HCV RNA at wk 24 compared to 0.59 +/- 0.1 in nonresponders, p = 0.00006). The magnitude of the decline in the viral load during the first 24 hours correlated with the likelihood of viral clearance at week 24. All of the individuals with a profound decrease in the viral load in the first 24 hours (at least a 1.4 log) cleared virus at Week 24. Seventy two percent of the individuals with a more modest decrease in viral load (0.8 - 1.39 log) cleared virus at Week 24, and only 35% of the individuals with a minimal decline in viral load (< 0.8 log) cleared virus at Week 24. Among those with a modest decline in viral load (0.8 - 1.3 log), the Week 24 clearance rate was significantly decreased among those individuals with more severe scaring in the liver.
The authors concluded that the HCV RNA decline during the first 24 hours after therapy initiation appears to be a good predictor of the likelihood of HCV RNA below assay detection at Wk 24. We anxiously await the SVR results to determine whether the decline in the viral load might be a useful predictor of the likelihood of a sustained virologic response. One day we might base the decision of whether to proceed with therapy on the viral load decline after 24 hours.
4) Amantadine Therapy for Chronic Hepatitis C: A Randomized Double-Blind Placebo Controlled Trial. Abstract #83
Several therapeutic agents are being evaluated for their ability to increase the SVR beyond that achieved with pegylated interferon and ribavirin. Amantadine has been evaluated in several trials as a possible adjunct to pegylated interferon and ribavirin. (Please see NATAP Conference Summaries for ICAAC, December, 2001 and AASLD, November, 2001 for a description of other studies that evaluated the efficacy and safety of amantadine in chronic HCV). The hypothesis underlying the use of this agent is that it might decrease liver inflammation. The goal of the current trial was to assess the safety and efficacy of oral amantadine for the treatment of chronic hepatitis C.
Fifty percent of the subjects included in this study were IFN nonresponders and fifty percent were not eligible for IFN treatment. They were excluded if they had received antiviral medications for other diseases besides hepatitis C, they were being treated with immunosuppressive therapy, or they were on anticoagulation. Subjects were randomized to receive: 1) amantadine 100 mg. twice a day or 2) placebo, and intent-to-treat analyses were performed at 6 and 12 months. Subjects were classified as to whether they were complete responders, partial responders (defined as at least a fifty percent decrease in ALT and HCV RNA) and nonresponders. Randomization was conducted according to age, gender, baseline HCV RNA level, HCV genotype, the severity of liver histology, and whether they had been treated with interferon in the past. Amantadine was administered for either six or 12 months. Children were also included in this study and they were treated with 4.4 mg/kg/d of amantadine not to exceed 150 mg per day.
A total of 41/152 (27%) individuals (including 7 children) responded to amantadine with loss of HCV RNA and normalization of ALT (p = 0.03). The overall SVR was 15.6%. An increased likelihood of an SVR was predicted by: 1) female gender, 2) low HCV RNA, 3) severe liver histology, 4) genotype 1. Amantadine had no effect on liver histology. There were no significant differences in the side effects between the two therapies, and the most common side effect of amantadine was fatigue. Other common side effects included depression (1.3%), impotence (0.7%), and weight loss (0.7%).
The authors concluded that oral amantadine is safe and effective compared to placebo. The SVR is comparable to that observed with IFN monotherapy. However, amantadine has fewer side effects. Amantadine may be a useful alternative to IFN particularly among those individuals who are not able to tolerate interferon including those with significant depression, low platelets, low white blood cell count, and interferon failures. (editorial note from Jules Levin: A number of studies looking at amantadine have been conducted with very mixed results. Most researchers I speak with di not feel amantadine has any benefit).
5) -Pegylated (40 kDa, Branched) Interferon Alfa-2a (Pegasys) and Ribavirin (RIBA) in IFN-naive Patients with Hepatitis C and Advanced Fibrosis/Cirrhosis: Interim Results of a Randomized, Controlled Trial. Abstract #84
Although a SVR = 30% has been observed in Pegasys monotherapy trials among individuals with advanced scaring in the liver, few patients with advanced scaring have been included in studies which used pegylated interferon in combination with ribavirin. In other studies, individuals with advanced liver scaring have not responded as well as those with more mild liver disease. The goal of this study was to evaluate the efficacy of Pegasys in combination with two different RBV doses (1000-1200 mg. vs. 600/800 mg per day) in HCV-infected subjects who have advance scaring in the liver and who have never been treated with interferon.
In order to be included in this trial, subjects had to have advanced liver disease seen on biopsy (Metavir F3-F4) and to have compensated liver disease (as determined by a score of less than 8 on the Childs-Pugh scoring system). Subjects were treated with Pegasys (180 mcg SQ per week) and received standard RBV or low dose RBV, which were further divided based upon the individuals' weight (< 75 kg: 1000 mg or > 75 kg: 1200 mg) or (<75 kg: 600 mg. or > 75 kg: 800 mg per day). Subjects were treated for a total of 48 weeks with 24 weeks of follow-up.
Interim analysis was presented on 61 patients who have received 24 weeks of therapy, 28 who received standard dose RBV and 33 who received low-dose RBV. There was no significant difference in the age, body mass, or HCV viral load between the two groups at baseline. Overall, the Week 12 response was 79% vs. 67% and the Week 24 response was 93% vs. 82% in those who received standard or low-dose RBV. Based on genotype, 92% of HCV genotype 2/3 cleared by Week 8 compared with approximately 50% of HCV genotype 1 who cleared by Week 24.
In terms of adverse events, there was a decrease in the average number of white blood cells that fight infection (neutrophils) from 3.3 to 1.6 cells/mm3 (less than 750 cells/mm3 is considered severe). The average number of platelets decreased from 154,000/mm3 to 97,000/mm3. There was also a decrease in the average hemoglobin level from 14.5 gm/dL to 11.7 gm/dL. Twenty-one subjects in the standard dose group and 6 subjects in the low-dose group had severe anemia (Hb < 10 gm/dL). Pegasys had to be held in 25% vs. 15% and RBV in 7% vs. 0% of the individuals in the standard dose compared to the low-dose groups, respectively. Medication had to be discontinued in 10% of the individuals because of depression (4), liver failure (1), overdose (1).
The authors concluded that nearly 90% of HCV-infected individuals with advanced fibrosis/cirrhosis treated with Pegasys interferon in combination with ribavirin will have HCV RNA below assay detection by 24 weeks of therapy. The response rate is increased with higher doses of RBV, which appears to be well tolerated. Clearance is delayed in those with HCV genotype 1 compared to those with genotype 2/3.
II. AASLD Research Forum: HCV Treatment Issues
The abstracts in this session dealt primarily with HCV treatment in "special populations"those with HIV/HCV coinfection, African Americans (who have been shown to have a decreased response to HCV treatment), illicit drug users, and individuals with normal ALT.
1) Pegylated Interferon Alfa-2b (PegIntron) Plus Ribavirin Versus PegIntron for Treatment of HIV/HCV Co-infected Patients (pts): An Open, Multicenter, Randomized Trial. Abstract #115
This study, which was presented by a group from Italy, was undertaken because the authors realized that the percentage of deaths from liver disease among HIV-infected individuals had increased from 5% in 1990 to 38% in 2000. This study enrolled subjects who had never received treatment for HCV and who had HIV disease under reasonable control (CD4 count > 300 cells/mm3 and HIV RNA < 400 copies/ml). Subjects were treated either with pegylated interferon 1.5 mg/kg per week and RBV 800 mg per day or pegylated interferon monotherapy.
A total of 59 individuals received combination therapy and a total of 63 individuals were treated with monotherapy. At baseline, there were no significant differences in the age of the two groups. The genotype distribution was as shown below:
|
|
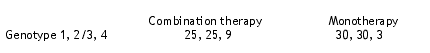 |
| |
The 24-week responses were presented. The overall response rate was 34.7%. Among those who received combination therapy, 14/25 (56%) subjects responded. Among those who received peg-IFN monotherapy, the response rate was 3/24 (12.5%) (p = 0.002). Consistent with other studies, those individuals who had at least a two-log decrease in HCV RNA during the first three months were more likely to be responders. A total of 35/122 (28.9%) of the individuals discontinued therapy during the study, most during the first month of therapy. Common reasons for discontinuation of medication included flu-like symptoms, depression, and hematological toxicity. The mean reduction in CD4 cells was from 581 to 452 during the 24 weeks of the study.
This study shows that the 24-week response rate is very high in coinfected patients. The participants in this study also had a very high drop out rate, higher than in other studies of coinfected individuals. During the question period, the presenter was asked whether the nurses who administered the medications had special training in hepatitis treatment, which did not seem to be the case. The point is that coinfected patients, who have many more comorbid diseases than other HCV-infected individuals, may need treatment by individuals who have special training in order to be able to stay on their medications.
2) Pegylated Interferon alfa-2b (PegIntron) and Ribavirin for the Treatment of Chronic Hepatitis C Infection in African Americans and Non-Hispanic Whites. A Preliminary Report. Abstract #116
The primary objective of this study was to evaluate whether the sustained virologic response (SVR) in African-Americans (AA) differs significantly from that observed in nonhispanic whites treated with pegylated interferon and ribavirin. One hundred AA and 100 Caucasian subjects with compensated cirrhosis were treated with pegylated interferon and RBV. The primary endpoint was the SVR. Secondary endpoints were end of treatment response (ETR), histologic improvement and quality of life (QOL).
Genotype 1 was present in 98% of the individuals in both groups. High blood pressure and diabetes were more common in the AA, while depression was more common in the Caucasians. The 12-week virologic response was 58% in Caucasians and 12% in AA (p < 0.001), and the 24-week virologic response was 62% in Caucasians and 22% in AA. 69% of the Caucasians had a 2-log decrease in HCV RNA compared to only 40% in AA. Discontinuation rates were 21% in Caucasians and 19% in AA.
This study shows that, independent of genotype, AA had a lower response rate than Caucasians. The precise reasons for the lower response rate are not known at this moment, but this topic will be the subject of a large, multicenter, NIH funded study that is set to begin very shortly that will evaluate this question.
3) The Impact of Intervening Substance Abuse on Hepatitis C Treatment Outcomes in Recovering Injection Drug Users: An Interim Analysis. Abstract #118
HCV-infection is a particular problem in illicit drug users. Depending upon the study, 60-90% of these individuals are HCV infected and 60% of the new cases of HCV occur in individuals who use illicit drugs. The goal of this study was to assess the impact of alcohol consumption, length of sobriety, and intervening drug use on treatment outcome with interferon/ribavirin.
To accomplish these goals, fifty-seven injection drug-users who were enrolled in a methadone maintenance program were treated with combination therapy consisting of interferon and ribavirin. These individuals had been infected for an average of 29 years and 61% of them had a history of depression.
The end-of-treatment response rate was 54% and 22% of the individuals withdrew from the study. Those individuals who did not use alcohol showed a trend toward an improved response rate compared to those who ingested alcohol during treatment. The ETR in those who ingested alcohol was 42% compared to 67% in those who did not ingest alcohol (p = 0.13). Individuals who continued to use illicit drugs demonstrated a trend toward a reduced ETR of 36% (p = 0.15) while the ETR did not differ significantly in those individuals with either < 6 months of abstinence or in those individuals with more extended abstinence (36% vs. 56% vs. 62%, respectively). Factors that were associated with an increased likelihood of an ETR included no or minimal drug use, extended or short (< 6 months) duration of sobriety, and no alcohol use.
This study suggests that cessation of drug and alcohol use, even for a short period of time, can improve the likelihood of compliance with anti-HCV medication and that alcohol and illicit drug use may have a negative impact on HCV treatment outcomes. Even modest alcohol and drug use can have an effect on the HCV SVR. Therefore, rapid intervention is needed in the case of individuals who are on anti-HCV treatment who return to drug or alcohol use.
4) Does Normal ALT Exclude Severe Hepatic Fibrosis in Patients with Chronic Hepatitis C? Abstract #120
ALT is an enzyme that is released from liver cells when they die. In the past, ALT was used as a primary marker of liver disease. HCV-infected individuals with persistently normal ALT are thought to have no or minimal fibrosis. Therefore, the issue of whether or not these individuals should be treated for HCV infection remains controversial. The goal of this study was to determine the prevalence of normal ALT at the time of first evaluation for liver disease and to determine the amount of inflammation and scaring in HCV-infected individuals with normal ALT.
Twenty-five out of 198 consecutive HCV-infected individuals had normal ALT. There was no difference in basic demographic characteristics including gender, age, duration of infection, and route of acquisition between those individuals with normal ALT values and those with persistently elevated ALT values. However, several other laboratory parameters including total bilirubin (p = 0.01), AST (p= 0.0005), serum iron (p = 0.003) and body mass index (p 0.03) were significantly lower in those individuals with normal ALT compared to individuals with persistently elevated ALT. There was no difference in the percentage of individuals with severe hepatic fibrosis (stage III or IV) comparing the two groups (high ALT 52/173 [30%] vs. normal ALT 6/25 [24%], p = 0.5). The amount of inflammation was significantly reduced in HCV-infected individuals with normal ALT compared to those with elevated ALT (p = 0.03). Only 18/25 had persistently normal ALT (i.e. these subjects had ALT values that occasionally were outside of the normal range).
Based upon this study, the authors concluded that the ALT values fluctuate considerably in HCV-infected individuals, so a value in the normal range should be followed up with additional measurements. The amount of inflammation in the liver and the body mass index are significantly decreased, while there was no change in fibrosis levels, in individuals with normal ALT compared to those with elevated ALT. Normal ALT should not preclude an evaluation for treatment and the consideration of a liver biopsy.
5) Interferon Alfa-2b and Ribavirin for Patients with Chronic Hepatitis C and Normal ALT: Final Results. Abstract #82
Since most treatment trials have excluded patients with normal ALT and interferon monotherapy studies have suggested that individuals with normal ALT do not respond as well to treatment, the aim of this study was to evaluate two doses of interferon in combination with ribavirin in individuals with normal ALT. This topic is of particular importance since between 5-20% of individuals with normal ALT have substantial scaring in the liver. The first NIH Consensus Conference in 1997 recommended that treatment of individuals with normal ALT should be considered investigational. Since there is limited data on treatment of individuals with normal ALT with combination therapy, this study was performed.
Chronically HCV-infected individuals with two normal ALT values at least three months apart were randomized to receive: 1) IFN 3 MU TIW plus RBV 1000-1200 mg per day (group 1) or 2) IFN 5 MU TIW plus RBV 1000-1200 mg per day (group 2). Subjects were treated for 48 weeks unless a Week 24 PCR was positive.
Fifty-six individuals received at least one dose of medication and 43 (77%) received at least 24 weeks of treatment. There were no significant differences in the age and gender of the subjects in the two groups. 69% of the individuals in group 1 and 81% of the individuals in group 2 were infected with HCV genotype
1. The overall response rate was 32%, 24% among the genotype 1 subjects and 80%
among the genotype 2 subjects. There was no significant difference in the overall response rate between the two dosages (group I: 7/29 [24%], group 2: 10/27 [37%], p = 0.39). In terms of other factors that affect response, the amount of scaring in the liver was not a significant factor in the determination of a response. Only 1/10 African-American patients had a response. In terms of side effects, they were not significantly different from treatment trials using combination therapy in other categories of HCV-infected patients. ALT elevation in response to the medication was not a significant problem in these subjects.
The authors of this study concluded that the SVR rates were roughly similar between subjects with normal and elevated ALT. The higher dose of IFN appeared to be more efficacious in genotype 1 patients. The most recent recommendations from the NIH Consensus Conference held in June 2002 are that HCV-infected individuals with normal ALT should be evaluated for therapy, even though it is now widely accepted that the rate of scaring may be reduced in comparison with patients with abnormal ALT.
III. AASLD Research Forum: Viral Hepatitis and Liver Transplantation
1) Interferon Alfa-2b and Ribavirin vs. Placebo as Early Treatment in Patients Transplanted for Hepatitis C End-Stage Liver Disease: Results of a Multicenter, Randomized Trial. Abstract #199-
The goal of this study was to evaluate whether interferon a-2b and ribavirin initiated 14-28 days after liver transplantation decrease the recurrence rate of HCV. Subjects were randomized to receive IFN/RBV or placebo. IFN was initiated at 1.5 MU SQ three times per week and increased to 3 MU three times per week after 2 weeks. RBV was initiated at 400 mg per day and the dosage was increased to 200 mg per day in increments every 2 weeks until subjects reached a maximum of 1000 mg per day.
A total of 32 patients participated in the study, 21 in the treatment group and 11 in the control group. The SVR was 3/21 (16%) in the treated group compared to 0/11 (0%) in the control group. Anemia occurred in 12/21 of the patients and 10 had to discontinue RBV or had to have dose reductions. Nine patients required blood transfusions. One patient died 506 days after liver transplantation and one patient had severe recurrent HCV while on placebo. Subsequently, he was treated with open label IFN/RBV and achieved an SVR.
The goal of this study was to see whether interferon and ribavirin given 10-20 days after liver transplantation could prevent recurrence of severe hepatitis C. While the authors were able to achieve an SVR in 16% of the individuals, there is a significant degree of toxicity associated with the regimen. If the subjects in this study had been able to tolerate the full dose of the medications, the SVR might have been improved. Since the side effect profile of pegylated interferon is similar to standard interferon, this medication probably will not have a substantial role in the treatment of HCV infected individuals post transplant. Newer therapies, which are currently being tested in individuals pretransplant and which may be less toxic, will need to be tested in individuals post transplant.
2) Defining the Role of HCV-Specific T-Lymphocytes in Hepatitis C: Recurrences after Liver Transplantation (LT). Abstract #201-
Immune responses against HCV are important to prevent disease recurrence after liver transplantation. This study investigated whether T cell responses are important in preventing HCV recurrence after liver transplantation. Both CD4 (helper) and CD8 (killer cell) activity was assessed in this study. 85% of the patients had responses to at least one of the HCV proteins. Individuals with more advanced disease had T cell responses to two regions of the HCV genome (core and nonstructural region (NS 5) compared to those who had less advanced disease. Only 24% of the patients had a CD8 response. The authors concluded that those individuals who have stronger CD4 responses after liver transplantation might be more likely to have worse disease recurrence.
3) Moderate to Severe Recurrent Hepatitis C after Liver Transplantation in Older-Donor Organ Recipients. Abstract #202-
All HCV-infected patients who have a liver transplant (LT) have recurrence of the virus after the transplant, and the scaring in the liver is usually more severe and occurs more rapidly. This study evaluated the effect of age on clinical and virologic outcomes after liver transplantation. A single pathologist evaluated the amount of inflammation, scaring, and fat in the liver. Severe liver inflammation was defined as 1) the presence of a large number of inflammatory cells in the liver seen under the microscope (hepatic activity index > 9), 2) Liver scaring with a score > 3, or 3) the presence of inflammatory cells with other microscopic changes (i.e. a significant amount of bile present in the bile ducts referred to as cholestatic hepatitis).
Sixty-three individuals underwent LT, 15 individuals were older than 65 years of age and 48 were younger. Eight patients died during the first year after the LT, the majority (6) from causes unrelated to HCV. Two patients died from cholestatic hepatitis (a type of hepatitis where the bile ducts become clogged). Recurrent hepatitis C requiring treatment (47.9% vs. 40%) and moderate to severe HCV requiring treatment (53.3% vs. 16.7%) occurred more frequently in the older subjects compared to the younger subjects.
The authors concluded that since moderate to severe recurrent hepatitis is more frequent in older age liver donors, organs from these individuals should be used cautiously in individuals who require transplantation for HCV related disease.
4) Complete Withdrawal of Immunosuppression in Liver Transplant Recipients with Recurrent Hepatitis C: Safety and Short-Term Outcome. Abstract #203
This study evaluated the effect of withdrawing immunosuppression in HCV-infected subjects who underwent LT. Immunosuppression is necessary because most patients have acute recurrence of HCV after liver transplantation. At one year, 55% have chronic hepatitis and at 5 years, 10% will have recurrence of cirrhosis. One important unanswered question is why does cirrhosis recurs more quickly after LT than in the native liver. The aim of the current study was to evaluate whether immunosuppression can be withdrawn in liver transplant recipients. The rationale behind the study is that immunosuppression may contribute to accelerated hepatic fibrosis.
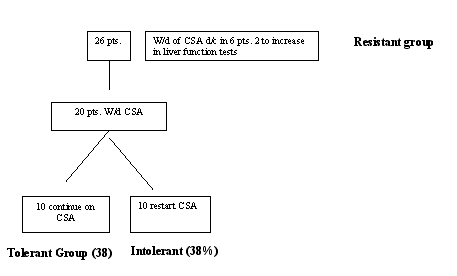
26 subjects who were HCV RNA+, who had elevated ALT and who had undergone LT were evaluated. All of the subjects were on maintenance cyclosporine (CSA) a drug frequently used to maintain immunosuppression after LT. 21/26 (81%) of the subjects had been treated with interferon and ribavirin at some point in the past. All subjects were started on a slow taper of CSA. The table below demonstrates the schema of the study:
|
|
 |
| |
Compared to the other groups, members of the tolerant group were followed for a longer time after transplant and had lower basal viremia. After withdrawal of immunosuppression, these individuals had no change in their liver function tests, and liver biopsies at the time of discontinuation of immunosuppression showed no evidence of rejection. A follow-up liver biopsy performed one year after the withdrawal of immunosuppression was unchanged from the earlier one. Those in the resistant group had an increase in LFTs very shortly after withdrawal of immunosuppression without a marked change in viremia. The liver biopsy in these individuals revealed acute rejection and all of them were restarted immediately on immunosuppressive treatment. Those individuals in the intolerant group had liver biopsies that revealed acute rejection and all were restarted on immunosuppression without adverse sequelae.
Overall, I think that this is one of the strongest studies related to liver disease presented at DDW. It demonstrates that withdrawal of CSA is feasible in almost 40% of the individuals. A lower HCV viral load and a longer time of follow-up after transplantation may be useful markers to predict who might be able to tolerate withdrawal of immunosuppression. If the trial of immunosuppression withdrawal is not successful, CSA can be rapidly restarted without adverse sequelae. During the question and answer period, the investigators indicated that they plan to follow up on this study with a randomized trial.
5) Early Treatment of Recurrent Hepatitis C in Liver Transplant Recipients: Is the Virological and Histological Response Better? Abstract #204-
The aim of this study was to evaluate whether administration of anti-HCV treatment within the first three months following liver transplantation will result in improved histologic and virologic responses. Participants in this study received IFN 3 MU three times per week and RBV 400 mg twice a day within the first three months after liver transplantation and were compared to subjects who were treated after three months (i.e. early vs. late treatment after liver transplantation). A total of 59 potential subjects were screened and 33 were enrolled, 17 in the group that was treated within the first three months after liver transplant and 16 in the group that was treated more than three months after liver transplantation. The major reasons for exclusion from the study included: depression, acute rejection of the transplant, and low hemoglobin or white blood cell counts. Of the treated patients, 7/17 and 6/16 were able to complete the one-year course of therapy. Sixty-two percent of those in the early treatment group and 38% of those in the late treatment group had either no change or improvement in fibrosis, although the small numbers of individuals precluded a finding of statistical significance between the two groups. Unfortunately, however, progression of fibrosis was observed in 50% of the individuals despite treatment. Overall the study demonstrated that there was no difference in the response between early and late treatment for HCV.
In summary, this study demonstrates that many individuals who have undergone liver transplant are excluded from treatment for HCV; that even if treatment is administered, a large percentage are not able to tolerate treatment; and despite treatment, fifty percent of the individuals have histologic progression while on treatment. The identification of individuals post hepatic transplantation who might be appropriate for HCV treatment will necessitate further evaluation.
IV. Miscellaneous
Sampling Variability on Percutaneous Liver Biopsy in Patients with Chronic Hepatitis C Virus Infection. Abstract #117-
The goal of this study was to determine the sampling variability on percutaneous liver biopsy obtained from two separate sites. Twenty chronically HCV-infected individuals without other types of liver disease underwent two passes through the same insertion site. However, the biopsy needle was rotated 30-45 degrees between the two passes so that each sampled different parts of the right lobe. A single pathologist reviewed each biopsy and Knodell's Histological Activity Index (0-22) was used to assess the amount of inflammation (0-18) and fibrosis (scaring) stage (0-4).
Adequate specimens were obtained in 19 individuals. The mean difference between the two biopsies in HAI score was 3.0 +/- 2.2 (range 0-9) for the HAI, 2.4 +/_ 2.2 (range 0-7) for inflammation, and 0.5 +/- 0.9 (range 0.3) for scaring (fibrosis). Thirteen (68.4%) of the subjects had a difference of > 2 and 9 (47%) had a difference > 3 both in the inflammatory score and in the amount of fibrosis. Six (31.5%) had a difference >1 and 3 (15.5%) had a difference in >2 in the fibrosis score. These three patients also had the highest degree of difference in the inflammatory score.
The authors concluded that there is considerable variability on percutaneous liver biopsies for assessment of the amount of inflammation and scaring. Since the biopsy only samples approximately 1/50,000 of the liver, these results seem to be reasonable. However, until better markers of fibrosis are available in the peripheral blood, the liver biopsy is still the gold standard for the assessment of liver disease and should be considered as a procedure to investigate the disease status in almost every HCV-infected individual.
V. AGA Poster Session-Clinical Hepatitis-Epidemiology and Pathogenesis
Follow-up of Patients with Spontaneous Clearance of Viremia after HCV Infection is Unnecessary. Abstract #M1446
The aim of this study was to determine the likelihood of reversion of HCV RNA after spontaneous clearance. A total of 107/1612 (6.6%) individuals were HCV RNA negative and HCV antibody positive. Those individuals who cleared HCV were younger. No recurrence of infection was seen after a mean follow-up of 39 months.
Commentary: This abstract demonstrates, as has been documented by several other investigators, that in HCV monoinfected individuals viral clearance during the chronic phase occurs exceedingly rarely, if ever. However, there have been a few published reports of HCV clearance as a result of effective immune reconstitution in HIV/HCV coinfected individuals who were treated with antiretroviral therapy who had an increase in the number of CD4 T cells.
Prevalence of and Risk Factors for HCV RNA Detection in HIV/HCV Co-infected Patients Who are Antibody Negative. Abstract #M1618
This study evaluated HCV prevalence and risk factors to determine why some HIV/HCV coinfected individuals have detectable HCV RNA while they don't have detectable HCV antibodies. The study also evaluated the ability of HCV antibody to determine who has chronic HCV viremia.
A total of 3736 patients of whom 2484 (66.5%) had HCV antibody measurements were included in this study. 796/2484 (32.1%) of the participants were HCV antibody positive. Of these 474 were both HCV RNA and antibody positive.
Compared to HCV RNA measurements, HCV antibodies were very sensitive (96.8%) but not very specific (43.4%) for the diagnosis of chronic HCV disease. If someone has a positive antibody, then there is an 86.6% chance that they have chronic HCV viremia, while if they are antibody negative, there is a 78% chance that they do not have HCV viremia. This study found no association between HCV viremia and female gender, age, ethnicity, HIV risk factors, mean CD4 cell number, mean CD4 cell percentage, HIV RNA level, ALT or the year of HCV antibody test.
In addition to this abstract, several articles have been published addressing whether HCV antibodies are sufficient to screen for the presence of HCV in HIV/HCV coinfected individuals. Most of these articles have concluded that the antibody test is sufficient as a screen and that individuals who test positive for the antibody should be tested for the presence of HCV viremia by measuring the level of HCV RNA. In addition, several professional societies have recommended that the antibody test be used as a screen for HCV in HIV-infected individuals. An additional reason for screening with the antibody is the issue is cost, as the assays for HCV RNA are much more costly than the antibody test.
Intrafamilial Transmission of Hepatitis C in Patients with Hepatitis C and Human Immunodeficiency Virus Coinfection. Abstract #M1456-
The issue of HCV spread among family members, particularly as a result of sexual intercourse, is an issue that has been evaluated by following couples in which one member is HCV-infected and the other is not. These studies have generally shown that HCV transmission among family members is quite low and that sexual intercourse is not a very effective means by which to spread the virus. This study aimed to evaluate whether infection with HIV increases the risk of HCV transmission among family members. In this study, 126 HCV-infected individuals (index cases) and 221 family members were enrolled between January 2000 and August 2001. Fifty-three of the HCV-infected and 87 of the family members were HIV-infected. Seventy-three index cases and 134 family members served as the control group. The same HCV genotype was identified in three family members with HCV alone and in 2 coinfected individuals. Since these individuals did not have any other identifiable risk factors independent of contact with the index case, the authors consider these cases to represent intrafamilial transmission of HCV. The authors concluded that intrafamilial transmission of HCV is rare and that it is not increased in the presence of HIV infection.
Commentary: This study has several weaknesses. 1) The study design is retrospective and the conclusions have been based on patients' self-report. 2) The small number of patients and cases that these authors observed make comparisons difficult. 3) The method that these investigators used to determine whether intrafamilial spread occurred, namely genotype concordance, is limited. A more sophisticated analysis would have been to sequence regions of the virus isolated from the family members to look for genetic differences between the virus isolates from different individuals.
Hepatitis C Virus RNA in Patients with HIV Infection. Abstract #M1617-
This study found that 12% (26/214) of HIV-infected individuals were HCV RNA negative despite positive HCV antibody at first presentation. Furthermore, the study found that HCV RNA levels in this population were stable over time and that female gender, ethnicity (Hispanic), and elevated AST levels were independently associated with HCV RNA negativity.
This study, which was conducted at a single site, addresses an important issue of whether the incidence of chronic HCV viremia is increased in HIV/HCV coinfected individuals compared to HCV monoinfected (See abstract M1446 in this report). As mentioned above, HCV is rarely, if ever, cleared during the chronic phase although there have been a few reports of HCV RNA clearance in coinfected individuals as a result of immune reconstitution with antiretroviral agents.
Viral Kinetics of Hepatitis C Virus (HCV) Genotype 3 Is Faster Than Genotype 1 HCV: Effect of Different Treatment Schedules. Abstract #M1606
The increased efficacy of anti-HCV therapy in HCV genotypes 2 and 3 compared to genotype 1 has been well documented. It has also been shown that the decline in genotype 2 is faster than the decline of genotype 1 (Neumann et al, JID, 2000). The goal of this study was to see if the decline in HCV RNA in genotype 3 is also faster than genotype 1.
In this study, 33 (23 genotype 1 and 10 genotype 3) HCV-infected patients were treated with one injection of interferon 9 MU x 1 day and viral decline was assessed. Subjects were then randomized to receive 3 million units every day or every other day for 14 days. Thereafter, they were treated with 3 MU per day. All patients also received ribavirin 1000-1200 mg per day.
The authors found that the decline in HCV RNA during the first 24 hours was significantly faster for genotype 3 compared with genotype 1 (log 1.78 + 0.5 vs. log 0.93 + 0.4, p = 0.001). The decline during the second phase was also faster for genotype 3 compared to genotype 1. The decline was also faster for those individuals who received the medication every day as compared to those who received every other day treatment. The more rapid decline of genotype 3 may be one reason why genotype 3 has an improved response to interferon.
Longitudinal Changes on Treatment Schedules for Hepatitis C Virus: Effect of Ribavirin on Early Kinetics. Abstract #M1608
This study was designed to assess the effect of the addition of ribavirin on HCV RNA decline. There was approximately a one-log increase in the decline in HCV RNA after the addition of RBV in three interferon naive, genotype 1 individuals. These investigators also treated previous IFN monotherapy nonresponders and relaspsers. They found that HCV RNA declined faster in the relapsers than in the nonresponders, which suggests that the rate of decline may be a useful characteristic by which to determine the likelihood of a virologic response.
Abnormalities in Cognition and Cerebral Metabolites in Individuals with Chronic Hepatitis C Infection in the Absence of Liver Cirrhosis. Abstract #M1611-
Many people who are infected with hepatitis C complain of brain fog, fatigue, depression, forgetfulness, and difficulty concentrating. HCV-infected people also have decreased quality of life. Therefore, the goal of this study was to investigate the problems in the brain that might lead to these symptoms.
A total of 44 patients were chosen to be studied, but 27 had to be excluded because of a history of intravenous drug use, current or prior alcohol abuse, cirrhosis, a prior course of interferon treatment, or a history of major depressive illness.
Seventeen subjects could be studied and they had a neuropsychological assessment including tests of verbal learning, memory, psychomotor function, speech, attention, fatigue, and depression. One half of the sample had a deficiency in verbal learning while psychomotor speed only impaired in a minority of the subjects. Five subjects underwent magnetic reasonance spectography, which suggested an organic cause for the impairments observed on neuropsychological testing.
A Simple Non-invasive Scoring System for Prediction of Severe Hepatic Fibrosis in Chronic HCV Patients Co-Infected with HIV. Abstract #M1621
The ability to predict the degree of liver scaring in HIV/HCV coinfected individuals without the need for a liver biopsy is very important and is an area of active research. Several groups have published abstracts and papers evaluating different laboratory tests that can be made on peripheral blood to avoid the need for a liver biopsy. This study evaluated a scoring system to predict liver scaring in HIV/HCV coinfected patients compared to HCV monoinfected patients. These investigators, who have previously found that the platelet count, AST, and the level of albumin are reliable predictors of liver scaring in HCV monoinfected individuals, wanted to evaluate if the same parameters were useful in coinfected individuals. They evaluated these parameters in 210 HIV/HCV coinfected individuals between 1996 and 2000. Fifty of the 210 (24%) individuals had severe scaring. Compared to HCV monoinfected individuals, these markers were neither very sensitive (78%) nor specific (87%) in coinfected individuals.
The markers that the authors evaluated, particularly the platelet count and albumin, are abnormal in end-stage liver disease (cirrhosis) after the development of decompensation. Cirrhosis is the end-stage of a several decade- long process of progressive liver scaring and refers to fibrous bands that surround the normal liver tissue. In the initial stages of cirrhosis, referred to as compensated cirrhosis, the liver can still produce the substances that it needs to perform its usual functions. As cirrhosis progresses, referred to as decompensated cirrhosis, the liver is no longer able to produce the substances it needs to in order to function. After the onset of decompensated cirrhosis, individuals develop symptoms of end-stage liver disease. As a consequence of the liver's inability to produce protein (albumin) in sufficient quantities, fluid will leak into body cavities where it does not belong, such as in the abdomen (referred to as ascites). An individual may also develop low platelets as a consequence of decompensated cirrhosis. While these may be good markers of end-stage liver disease, they may not be able to differentiate mild from moderate liver disease. In this case, a liver biopsy may be necessary. These markers, as have many others that have recently been investigated, are able to differentiate mild from end stage liver disease. Unfortunately, they are not useful in differentiating among milder forms of liver disease, which is the reason why liver biopsies are still important.
Does High Body Mass Index Affect Hepatic Fibrosis, Steatosis, and Inflammation in Patients with Chronic Hepatitis C? Abstract #M1632-
The presence of fat (steatosis) in the liver in HCV-infected individuals and the effect of fat on fibrosis progression are very important aspects of HCV disease that are receiving increasing recognition. Several recent reports have documented that HCV infection is associated with diabetes mellitus. It is also well documented that diseases such as increased body lipids (hypercholesterolemia), diabetes, obesity, and alcohol abuse can cause fat in the liver. However, the presence of fat in the liver by itself is not sufficient to cause fibrosis and progressive liver disease. Inflammatory cells, including lymphocytes and plasma cells, also appear to be a necessary requirement for the development of advanced disease. This study, which evaluated the association between higher body weight (as determined by the body mass index [BMI]) and advanced fibrosis, evaluated a total of 209 HCV-infected individuals. The authors found a significant association between a higher BMI and advanced fibrosis (stage III/IV) after controlling for diabetes and alcohol abuse. These findings suggest that controlling body weight may be beneficial in reducing the amount of fat in the liver and the progression to end stage liver disease.
Histological and Biochemical Differences between African-American and White U.S. Veterans with Chronic Hepatitis C: A Comparative Analysis. Abstract #M1614-
African-Americans (AA) constitute a significant percentage of HCV-infected individuals in the United States. For reasons that are not entirely clear, the response to conventional anti-HCV therapy (interferon and ribavirin) is significantly reduced in AAs, independent of genotype. This study evaluated pathologic differences between AAs and Caucasians with chronic HCV infection. A total of 105 individuals, 69 AA and 36 Caucasians, were included in the study. This study primarily evaluated the presence of fat in the liver (steatosis). Individuals in this study were divided into the following classification based on the degree of fat: 1) no fat (n = 37), mild fat (n = 30), moderate fat (n = 23), severe fat (n = 15).
The authors found that, in HCV-infected individuals, 1) increased fat in the liver is associated with increased liver necrosis and scaring (fibrosis) and 2) the degree of fat correlates with the ALT level. In untreated HCV infected individuals, worsening fat in the liver contributes to progressive liver disease. These authors did not find an association between fat in the liver and either portal or lobular inflammation. The mechanisms by which fat in the liver leads to increased scaring is currently an area of intense research. For the time being, individuals with fat in the liver are advised to seek the advice of a physician to guide them in controlling important cofactors such as diabetes, high cholesterol, obesity, etc. that might contribute to fat in the liver.
Long-term Outcomes of Hepatitis C Virus Related Compensated Cirrhosis: Clinical, Biological, and Histological Prognostic Factors of Decompensation, Hepatocellular Carcinoma and Death. Abstract #M1461-
One important area where further investigation is needed is why some people will develop scaring in the liver and will progress to end stage liver disease and liver cancer. Other people, who may have been infected with HCV for the same length of time, will not have any scaring in the liver after years of infection. This study followed 88 individuals with biopsy-proven, compensated cirrhosis who were infected between 1990 and June 2000 and attempted to identify the factors that are predictive of decompensation, liver cancer, and death.
The median time that people were followed in this study was approximately 4 years. The risk of developing decompensation was 22%, of developing liver cancer was 9.6% and death was 15%. When multiple factors were analyzed together, the presence of esophageal varices (dilated veins in the esophagus that develop as a result of cirrhosis) was predictive of liver decompensation and a low platelet count was predictive of the development of liver cancer and death. The authors did not find an association between histologic features and the outcome variables (decompensation, liver cancer, and death).
Characteristics of Patients with Chronic Hepatitis C and No Fibrosis (Stage 0). Abstract #M1469-
The factors that determine whether an HCV-infected individual will develop hepatic fibrosis (scaring) have not been determined. This study sought to identify clinical markers that might identify individuals who are at risk of developing liver scaring. This study was a retrospective chart review. The study group consisted of 27 patients with chronic hepatitis C who had no evidence of scaring on liver biopsy. These individuals were matched for duration of infection with individuals who had stage 4 scaring (advance liver disease consistent with cirrhosis). Younger age and lower grades of inflammation on liver biopsy were both associated with the absence of scaring in the liver. There was no association between alcohol consumption and genotype and liver scaring. The results of this study suggest that those individuals who acquire the disease at a younger age and those who don't have inflammation in the liver are less likely to develop scaring.
PRESENTATIONS DEALING WITH ALFA FETOPROTEIN
Two presentations at the meeting dealt with the subject of the effect of interferon on alfa fetoprotein (AFP).
Therapy with Interferon Decreases Serum -Fetoprotein (AFP) Levels in Individuals with Chronic Hepatitis C (HCV). Abstract #M1457
It has been shown previously that interferon decreases serum alfa fetoprotein (AFP) levels in subjects with chronic HCV. AFP is an enzyme that is produced in the fetal liver. High AFP levels can occur in cases of advanced liver disease (cirrhosis) or liver cancer. The upper limit of normal in adults in most laboratories is 10 IU/ml. Usually advanced liver disease will result in levels up to 100 IU/ml while liver cancer will result in levels greater than 100 IU/ml. The goal of this poster was to assess the effect of interferon on serum AFP levels.
A retrospective study enrolled 14 HCV-infected individuals with elevated AFP, 7 of whom were treated with interferon alone (n =1) or combination interferon and ribavirin (n = 6). Prior to treatment, the mean AFP level in the treated group was 112 IU/ml, which decreased to 40 IU/ml and 24 IU/ml 1 and 3 months after the initiation of therapy, respectively. Six to 12 months post therapy, the AFP level increased to 170 IU/ml. In the no therapy group, the mean AFP at baseline was 61 IU/ml, which increased to 91 IU/ml and 126 IU/ml, 3 and 6-12 months later. This study demonstrates that IFN, possibly as a result of the antiproliferative effect, can result in a decrease in AFP levels.
Alpha Feto-Protein Levels in Hepatitis C Subjects Treated with Combination Therapy: Abnormal Levels Decline with Therapy. Abstract #119
New measures of disease severity are needed in viral hepatitis. Markers such as AFP may be more indicative of the amount of liver scaring than markers that indicate the amount of liver inflammation. Viral hepatitis is one of the reasons for an increase in the AFP level. Therefore, these investigators measured AFP levels in individuals from three studies. 1) Individuals treated with standard interferon three million units three times per week, 2) individuals treated with daily interferon and ribavirin, and 3) individuals treated with pegylated interferon and ribavirin. The goal of this study was to evaluate the effect of combination therapy with IFN and RBV on AFP levels, to evaluate the change in AFP levels in individuals who clear the virus infection, and to evaluate the factors that are associated with increased AFP prior to beginning therapy. AFP levels were drawn at baseline and every three months while subjects were being treated.
A total of 231 subjects were enrolled in the study and the mean baseline AFP level was 8.5 IU/ml. Thirty-three individuals had elevated AFP levels (>10 IU/ml) that decreased to within the normal range in 24/33 (73%) after initiation of therapy. In those individuals who had an abnormal AFP level, there was a significant decline at three and six months. Those individuals who had cleared virus had more profound decreases in the AFP level, but even individuals who did not clear virus had a decrease in AFP. The type of interferon did not affect the degree of decrease in the AFP level. In terms of the predictors of elevated AFP levels at baseline, the amount of scaring and the genotype of the virus (genotype 1a) were associated with higher AFP levels.
The authors concluded that AFP may be an additional marker that should be followed in patients on treatment. A decrease in the AFP level in response to therapy may provide reassurance to patients that the treatment may be having an effect, even in the absence of a decrease in the viral load.
POSTERS DEALING WITH THERAPY
Maintenance Ribavirin for Patients with Chronic Hepatitis C Who Have Failed to Respond to Combination Therapy. Abstract #M1453-
The issue of how to treat individuals who have failed to achieve virologic clearance after a course of pegylated interferon and ribavirin continues to be a controversial area. Many doctors believe that patients who have significant fibrosis and who do not clear HCV using pegylated interferon and ribavirin should be treated with maintenance therapy. Most doctors currently recommend using interferon alone, however this trial investigated the use of ribavirin monotherapy (without interferon). In this study, patients were treated with standard interferon (3MU three times per week) and ribavirin 1000/1200 mg each day) for 24 weeks. After 24 weeks, those individuals who still had detectable HCV RNA were randomized to receive ribavirin alone or placebo for the next 48 weeks. Patients who responded remained on combination therapy for 48 weeks. All patients underwent repeat liver biopsy at 72 weeks.
A total of 108 individuals were treated with combination therapy and 50 still had detectable HCV RNA at 24 weeks. Of these individuals, 34 were randomized to receive either ribavirin or placebo. Among the individuals who received ribavirin alone, the amount of inflammation decreased significantly compared to those individuals who received placebo while the amount of fibrosis did not change. So although RBV had no effect on fibrosis, the drug did cause a reduction in the amount of inflammation. These results are quite interesting and should be the subject of additional studies. Several large clinical trials are currently being performed that are testing different maintenance therapy regimens. The optimal management for those individuals who do not achieve a sustained virologic response is currently an area of intense investigation.
Open-Label Rising Dose Study of Omega Interferon (w-IFN) in IFN-Naive Patients with Chronic Hepatitis C. Abstract #M1454-
Interferon alfa is the most common type of interferon used to treat hepatitis C virus infection. However, even with the conjugation of interferon alfa to polyethylene glycol, pegylated interferon, the medication is only effective in approximately 50% of individuals. This study evaluated another interferon, omega interferon, for its effect on HCV RNA. Week 12 data were presented.
The authors found that among IFN-naive patients treated for 12 weeks with omega interferon, HCV RNA levels were undetectable in 46/90 (51%) of patients of all genotypes and in 19/55 (35%) of patients with genotype 1 infection. Omega interferon has a safety and tolerability profile similar to that of other interferons including influenza-like illness and thrombocytopenia that appear to be dose related. However, whether omega interferon will lead to a substantial improvement in the treatment of HCV over pegylated interferon alfa in combination with ribavirin will necessitate further investigation.
|
|
| |
|
 |
 |
|
|