 |
 |
 |
| |
INTERFERON GAMMA-1B FOR THE TREATMENT OF CHRONIC HEPATITIS C INFECTION
Reported by Jules Levin
|
| |
| |
Don Rockey reported preliminary data from a study of an antifibrotic drug at the DDW conference.
Despite improvements in response rates for chronic hepatitis C virus (HCV) infection, many patients will not respond to standard therapy or have contraindications. Given the complications of HCV are due to fibrosis and ultimately portal hypertension, an alternative strategy would be to target fibrosis. We previously found that interferon-gamma has potent anti-fibrotic effects on stellate cells. Thus, the aim of this study was to determine the safety and efficacy of interferon gamma-1b in patients with HCV. It is not expected that Interferon-Gamma would decrease HCV viral load, only that it might slow or regress fibrosis, liver disease. An anti-fibrotic drug would be for patients that are unable to achieve an antiviral response to Peginterferon plus ribavirin until more effective treatmemts for HCV are available.
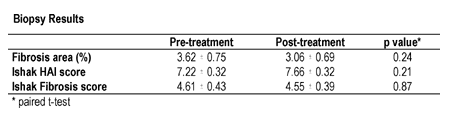
Twenty patients with chronic HCV who failed previous interferon-alfa based regimens or were intolerant to interferon-alfa therapy were enrolled. All patients received 200 mcg of interferon gamma-1b (Actimmune, Intermune, Brisbane, CA) subcutaneously three times weekly for 24 weeks. Patients underwent liver biopsy prior to and at week 24 of treatment. Biopsies were evaluated by a single blinded pathologist using the modified Knodell scoring system of Ishak. Fibrosis was quantitated by morphometric analysis after Sirius Red staining of specimens. The primary endpoint was a reduction of 1(absolute percentile) in fibrosis area by morphometric analysis. The study results showed Interferon-gamma did not change HCV-RNA.
The study population was 75% male and 70% Caucasian. Mean age was 47.9±7.5 years. All were genotype 1. Eighteen of 20 patients completed therapy. One discontinued therapy at week 1 due to constitutional symptoms; one discontinued at week 4 due to an increase in liver enzymes to greater than 2X baseline. No dose reduction was required. No serious adverse events occurred. Liver biopsy results are presented in the table. By morphometric analysis, 6 patients (30%) had a reduction in fibrosis area greater than 1 (absolute %). Just by coincidince or the fact that fibrosis can measurement can be variable in patients these number that showed improvement are not adequate. However, 3 (15%) had greater than 1% increase in fibrosis area.
The authors concluded that Interferon gamma-1b therapy is safe and well tolerated in patients with chronic HCV infection. Although there was no reduction in fibrosis overall, interferon gamma-1b led to a reduction in fibrosis in selected patients. The small sample size, potential for sampling error, and short duration of treatment limit our ability to conclusively comment on efficacy. However, these data provide a basis for further study of interferon gamma-1b in patients with chronic fibrosing liver disease. Supported by a grant from NIDDK. Interferon gamma-1b provided by Intermune.
In speaking with Intermune representatives at this conference they are committed to Interferon Gamma studies and larger studies are in progress. Although the data from this study was not impressive the study is only 24 weeks. In addition, there are other anti-fibrotoc agents in research.
|
|
 |
|
 |
 |
|
|