 |
 |
 |
| |
FUZEON: PATIENT ACCEPTANCE WITH SELF-INJECTION OF ENFUVIRTIDE (Fuzeon) FOR HIV OVER 48 WEEKS OF TREATMENT
|
| |
| |
Poster at 9th European AIDS Conference, Warsaw, Poland, October 2003
Cohen C1, Green J2, Wintfeld N2 and Patel K2. 1 Community Research Initiative of New England, Boston, MA, USA; 2 Roche, Nutley, NJ, USA
Reported by Jules Levin
This study evaluated patient acceptance of self-injection and how it affected them. The study authors used validated survey instruments to evaluate patients 660 patients in the TORO 1 and 2 phase III pivotal studies which compared Fuzeon plus optimized background therapy to optimized background therapy without Fuzeon. Fuzeon is a twice daily self-injection similar to taking insulin. Therefore, this study was performed to assess the patient experience of self-injecting. Here are some of the questions patients answered in the survey: “How Easy or difficult were the following for you?”, “How much have injections limited your ability to perform the following daily activities?”, “How much have injections limited your ability to perform the following daily activities?”. Below, you’ll find tables and pictures of the survey responses and an evaluation over the course of 48 weeks to see if patients became more comfortable with the injections. You’ll see that the study finds most patients, somewhere between 75-90% find various aspects of self-injection very easy or easy. 60-75% of patients report injections did not interfere with daily activities, and about 30% said it interfered some of the time. The study results suggest that over time some patients get used to self-injection and it becomes more routine. I think you’ll find the study interesting. This report will be posted & archived on the NATAP website for further reference, along with all pictures and tables, and along with other Fuzeon reports from EACS.
Introduction
Enfuvirtide (T-20) is a 36 amino-acid synthetic peptide inhibitor of gp41-mediated HIV-1-cell fusion. Recently approved in countries including the USA, EU and Switzerland, enfuvirtide demonstrates potent activity against HIV-1 isolates resistant to all three of the conventional antiretroviral classes.
As a peptide, enfuvirtide is formulated for twice-daily (BID) parenteral self-administration by the subcutaneous route. This unique mode of administration in antiretroviral therapy may result in patient anxiety over the process of preparing and self-injecting enfuvirtide, and over the possible impact of regular self-injection on their appearance and daily activities. Thus there is a need for a standardized procedure to assess patient acceptance of enfuvirtide and its effects.
The Subcutaneous Injection Survey (SIS) instrument is a previously-described self-assessment questionnaire developed to evaluate patient attitude to self-injection and related procedures. Factor analysis identified three unique subscales of treatment acceptance. A further three independent items mapped to multiple factors but were retained due to their potential importance. The SIS has been shown to have acceptable internal consistency by psychometric analysis and was available in all study languages, with standard translation and cultural validation methods used to assure conceptual equivalence.
We have previously applied the SIS to the combined TORO 1 and TORO 2 multinational Phase III trials of enfuvirtide to obtain patient acceptance data for short and medium-term use at 8 and 24 weeks, respectively. Here, we present 48 week SIS data from these two combined trials and assess patterns of change over long-term enfuvirtide use.
STUDY OBJECTIVE is to survey and assess the experience of patients self-administering enfuvirtide by BID subcutaneous injection at week 48 in the ongoing TORO 1 and TORO 2 Phase III clinical trials, and whether this experience changes significantly from earlier timepoints.
Methods and Patients. TORO 1 and TORO 2 are open--randomized, active-controlled, parallel group studies comparing the efficacy and safety of enfuvirtide (90 mg BID) in combination with an optimized background regimen (OB) with that of OB alone. If patients met protocol-defined virologic failure they were permitted to switch from OB alone to OB + enfuvirtide, with re-optimization of the OB, from week 8 onwards.
All patients had prior experience and//documented resistance to all three conventional drug classes, plasma viremia at least 5000 HIV-1 RNA copies/mL, and had been on a stable antiretroviral regimen (or no regimen) for at least 4 weeks prior to study entry.
In total, 661 patients were initially randomized to the enfuvirtide + OB arm and received at least one dose of enfuvirtide and at least one efficacy measurement. Of these, 618, 581 and 492 were surveyed using the SIS at weeks 8, 24 and 48, respectively. Patients who switched to enfuvirtide after initial randomization to OB alone were not surveyed.
The majority of patients enrolled to receive enfuvirtide in the combined trials were white (90%) and/or male (90%). Homosexual contact was the most likely route of infection (65%). Mean age at baseline was 42.4 years (SD = 7.9).
STUDY DESIGN. TORO 1 and 2 had very similar research protocols and enrolment criteria. SIS data from these two studies were therefore pooled for analysis.
The 18 item responses are measured on a five-point categorical response (Likert) scale. The SIS was completed by patients before any clinical procedures or consultations. Overall subscale scores are calculated as the average of individual item responses. Lower scores indicate less interference with activities or less difficulty with injection preparation. The statistical significance of changes in the distribution of responses to individual items over 48 weeks was assessed by Chi-squared analysis.
RESULTS
Of those who remained on-study and were available for survey with the SIS, 99.7% (616/618), 99.3% (577/581) and 99.8% (491/492) responded at weeks 8, 24 and 48, respectively.
Itemized SIS responses at weeks 8, 24 and 48 are shown in Figures 1–4.
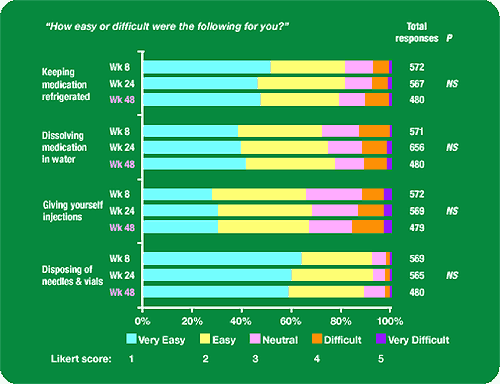
Figure 1. ease of injection subscale:
“How Easy or difficult were the following for you?”
Keeping medication refrigerated: about 50% found it very easy, 30% easy, 10% neutral, 5-10% difficult, 2% very hard.
Dissolving medication in water: about 40% found it very easy, 40% easy, 10% neutral, 5-15% difficult, 1-2% very difficult.
Giving yourself injections: about 30% very easy, 45% easy, 20% neutral, 10-20% difficult, 2-4% very difficult.
Disposing of needles & vials: about 60% very easy, 30% easy, 5-10% neutral, 2% difficult, 1% very difficult.
|
|
| |
| |
 |
|
| |
| |
Ease of injection subscale
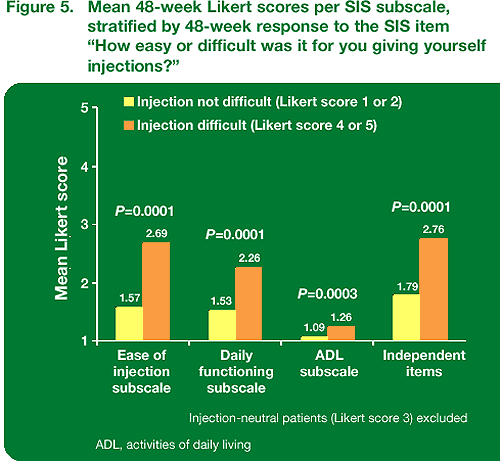
Patients who found giving themselves injections difficult (Likert 4 or 5) at week 48 showed significantly higher mean scores for all three subscales and the independent items (Figure 5).
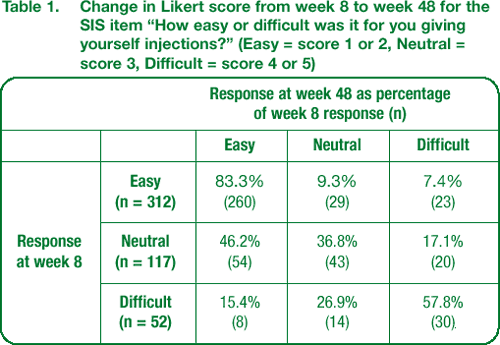
The majority of patients who found giving themselves injections very easy or easy at week 8 continued to find it easy at week 48 (Table 1).
The overall distribution of scores for ease of giving injections did not change from week 8 to week 48. However, a trend was noted towards patients finding injection easier over time. Forty-six percent of 117 patients who assessed self-injection as neutral at week 8 found it easy at week 48. Forty-two percent of 52 patients who found injection difficult at week 8 were neutral or found it easy at week 48 (Table 1).
No significant association was seen between difficulty of administration and patient height, weight or body mass index (Table 2). A slightly higher age was noted for patients who found injection very easy or easy relative to those who found it difficult or very difficult. This is unlikely to be clinically relevant given that the difference (1.6 years) is less than the standard deviation of the overall study population.
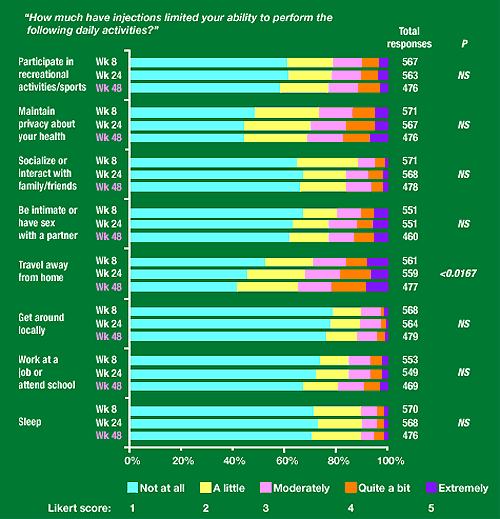
Figure 2. Daily functioning subscale
“How much have injections limited your ability to perform the following daily activities?”
Participate in recreational activities/sports: about 60% said not at all, 20% a little, 10% moderately, 5-10% quite a bit. 3-4% extremely.
Maintain privacy about your health: about 45-55% not at all, 20% a little, 15-20% moderately, 10% quite a bit, 5-10% extremely.
Socialize or interact with family/friends: about 65% said not at all, 25-30% a little, 5-10% moderately, 3-5% quite a bit, 1-2% extremely.
Be intimate or have sex with a partner: about 60-65% not at all, 15% a little, 10% moderately, 5-10% quite a bit, 5-10% extremely.
Travel away from home: 40-50% said not at all, 20-25% a little, 15% moderately, 5-10% quite a bit, 10% extremely.
Get around locally: about 75% said not at all, 15% a little, 10% moderately, 2-3% quite a bit or extremely.
Work at a job or attend school: about 75% said not at all, 10% a little, 7-8% quite a bit, 2-3% quite a bit, 2% extremely.
Sleep: 70% said not at all, 20% a little, 5% moderately, 2-3% quite a bit, 1% extremely.
|
|
| |
| |
 |
|
| |
| |
Figure 3. Activities of daily living subscale
“How much have injections limited your ability to perform the following daily activities?’
Prepare meals: 90% said not at all, 5-8% a little, 4% moderately/quite a bit/extremely.
Take baths or showers: 90% said not at all, 5% a little, 2% moderately, quite a bit/extremely.
Use the toilet: 95% said not at all, 5% moderately.
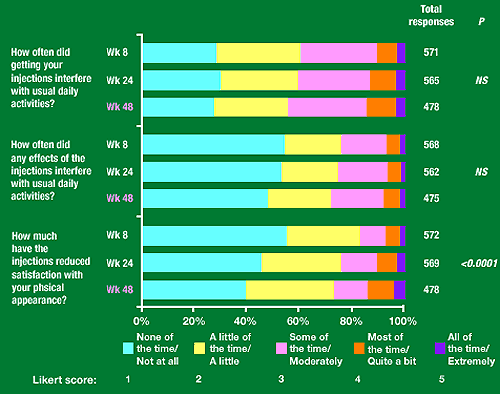
Figure 4. SIS Independent items
How often did getting your injections interfere with usual daily activities: 30% said none of the time/not at all, 30% a little of the time/a little, 25% some of the time/moderately, 5-12% most of the time/quite a bit, 4% all of the time/extremely.
How often did any effects of the injections interfere with usual daily activities: 50-55% said none of the time/not at all, 20% a little of the time/a little, 20% some of the time/moderately, 5-7% most of the time/quite a bit, 2% extremely/all of the time.
How much have the injections reduced satisfaction with your physical appearance: 40-50% none of the time/not at all, 30% a little of the time/a little, 10-12% some of the time/moderately, 5-15% most of the time/quite a bit, 3-4% all of the time/extremely.
|
|
| |
| |
 |
|
| |
| |
In general, the mean scores on the SIS subscales remained low at 48 weeks, indicating good acceptance of self injection. Mean scores ranged from 1.1 (activities of daily living) to 2.0 (ease of injection). The mean score of the independent items at 48 weeks was 2.1.
Of the 18 SIS items, the distribution of Likert responses over 48 weeks changed significantly only for travel away from home (daily functioning subscale) and satisfaction with personal appearance (independent item).
Mean score for impact on travel away from home increased from 2.0 at week 8 to 2.2 at week 48. Mean score for reduction in satisfaction with patient’s personal appearance increased from 1.7 to 2.1 over this period.
|
|
| |
| |
 |
|
| |
| |
 |
|
| |
| |
Discussion By Authors
In general, the mean scores on the SIS subscales remained low at 48 weeks, indicating good acceptance of self injection. Mean scores ranged from 1.1 (activities of daily living) to 2.0 (ease of injection). The mean score of the independent items at 48 weeks was 2.1.
Of the 18 SIS items, the distribution of Likert responses over 48 weeks changed significantly only for travel away from home (daily functioning subscale) and satisfaction with personal appearance (independent item).
Mean score for impact on travel away from home increased from 2.0 at week 8 to 2.2 at week 48. Mean score for reduction in satisfaction with patient’s personal appearance increased from 1.7 to 2.1 over this period.
Conclusions By Authors
Most patients in a clinical trial setting taking enfuvirtide for up to 48 weeks are satisfied with the ease of injection and impact on their activities of daily living. Acceptance of self-injection is not associated with baseline clinical characteristics.
Acceptance of self-injection and its effects is determined early and remains broadly constant over 48 weeks of use. Patients who find giving themselves injections difficult are more likely to report a negative impact on other aspects of daily living.
|
|
| |
|
 |
 |
|
|