 |
 |
 |
| |
New Atazanavir Information
Reported by Jules Levin
|
| |
| |
The Bristol-Myers Squibb Symposium at the Intl AIDS Conference in Paris just ended (6pm, Saturday July 12), where Kate Squires (USC) reported new information regarding atazanavir (ATV).
First, Squires discussed the interaction between tenofovir and ATV. In a study reported at the 2003 Retrovirus Conference ATV AUC and Cmin were reduced by about 25% when 300 mg of tenofovir was combined with ATV 300 mg plus 100 mg ritonavir (boosted PI regimen). It appears that tenofovir reduces ATV blood levels, so Squires suggested that when desiring to use tenofovir with ATV in treatment-naive patients, using boosted ATV (ATV 300mg/RTV 100mg once daily) will raise ATV levels well above needed levels. On average this should result in ATV levels 5 times above ATV unboosted levels. This recommendation is not yet FDA approved.
Squires addressed the results of BMS ATV Study 034, which compared ATV to efavirenz (EFV), each combined with AZT/3TC. In this study through week 48 BMS reported 32% of patients receiving ATV had <50 copies/ml (ITT) and 37% taking EFV had < 50 copies. Although the percent of patients who had <50 c/ml were about the same for both treatment groups, observers were confused as to why the viral response to EFV was so low and why it was lower than seen in other EFV studies. Regarding <400 c/ml, the previously reported data from Study 034 was that 70% of patients taking ATV had <400 c/ml and 64% of patients taking EFV had <400 c/ml at week 48 (ITT).
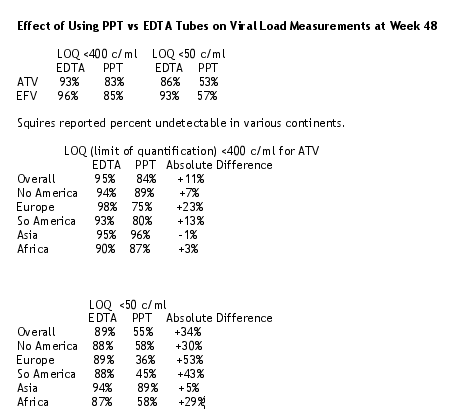
At the BMS Symposium Squires reported data related to this. She said that viral load samples in the BMS 034 Study were collected in PPT tubes and were spun and frozen in situ prior to shipping to a centralized lab. Additional samples were collected at study visits week 12, 24, and 48 in EDTA tubes (spun, separated, frozen, and were shipped from the study site). She presented new information on the effect of using PPT vs EDTA tubes on viral load measurements. Duplicated samples were assayed after collection in PPT or EDTA tubes. 584 patients were evaluable (300 on ATV, 284 on EFV): there were 805 patients in the main study at enrollment; 584 represents 88% of the 661 subjects treated for 48 weeks; 584 represents 73% of all 805 patients treated in the main study. Squires said the sample handling methodology affected the viral load responses.
|
|
 |
| |
|
 |
 |
|
|