 |
 |
 |
| |
|
Kaletra – Fosamprenavir Interaction Study
|
| |
| |
COMBINING GW433908 (FOSAMPRENAVIR) WITH Kaletra (LOPINAVIR/RITONAVIR) IN HIV-1 INFECTED ADULTS RESULTS IN SUBSTANTIAL REDUCTIONS IN AMPRENAVIR AND LOPINAVIR CONCENTRATIONS: PHARMACOKINETIC RESULTS FROM ADULT ACTG PROTOCOL A5143
Reported by Jules Levin
Summary by authors: Amprenavir and Lopinavir AUC12h and C12h are reduced when GW433908 and LPV/rtv are combined. There are no obvious RTV- mediated interaction: RTV concentrations comparable between groups. There is no apparent TDF-mediated interaction:
Although it is unclear whether lowered APV and LPV concentrations are inadequate for virologic response or response durability, based on these data, GW433908 + LPV/rtv does not appear to be a viable regimen in HIV-infected, treatment-experienced patients Since this lower drug exposure may put patients at risk of virologic failure, enrollment in A5143 was ended.
ABSTRACT
Background: A5143 was designed to evaluate the combination of GW433908 (908) + lopinavir/ritonavir (LPV/R), compared to 908/R alone or LPV/R alone with tenofovir (TDF)+1-2 NRTIs, in treatment-experienced patients. The lack of drug interaction data prompted an open-label, steady-state PK substudy to minimize subject risk.
Methods: A planned independent interim review occurred after the first 8 subjects were randomized to each arm. Arm A (n=8); LPV/R 3caps BID: Arm B (n=8); 908/R 700mg/100mg BID: and Arm C (n=17); LPV/R 3caps BID + 908 700mg BID. PK sampling (8 samples over 12h) occurred around observed doses at week 2-4. Samples were analyzed by LC/UV, and PK parameters (PKp) calculated by non-compartmental methods. A 1-sided t test on log transformed data was used with a Peto stopping boundary (p<0.001).
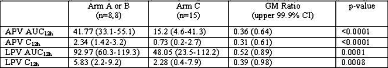
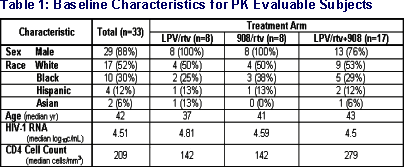
Results: Of 33 subjects, 29 were male, 21 white, with median age of 42y. Geometric mean (GM) (range) PKp are summarized below (C12h in mg/mL, AUC12h in mg◊hr/mL). RTV exposure was similar in all arms. TDF did not account for lowered PI exposure.
|
|
| |
| |
 |
|
| |
| |
BACKGROUND
A5143 is a multicenter open-label, randomized study of the virologic response of GW433908 (fosamprenavir, a phosphate ester pro-drug of amprenavir, APV) and lopinavir/ritonavir (LPV/rtv; Kaletra), in combination versus used alone in HIV-1 infected subjects with virologic failure.
Pharmacokinetic (PK) data was not available on GW433908 + LPV/rtv at time of study design
- steady-state interaction data between Agenerase and Kaletra in healthy volunteers (using 750 mg APV BID in combination with standard doses of LPV/rtv) demonstrated ß concentrations of both PIs
- this combination still produced PI concentrations considered clinically efficacious
Based on the Agenerase-Kaletra results and no interaction data between GW433908 + LPV/rtv, a PK substudy (A5147s) with real-time (within 2 weeks) analysis was designed to enroll the first 25 subjects randomized to each arm of A5143
Prior to A5143 enrollment, GSK shared preliminary results from two healthy volunteer studies suggesting a significant 908-Kaletra® interaction
- since the GSK data could not be definitively extrapolated to the A5143 population (healthy volunteer data with different doses of PIs than A5143), and the team decided to proceed with the study
To minimize participant risk, an interim analysis was planned to evaluate drug concentrations between the study arms from the first 8 evaluable subjects in each arm.
Additionally, the first 36 subjects enrolled in A5143 were required to have HIV susceptible to both APV (<2 fold) and LPV (<2.5 fold), based on a Virtual Phenotype.
STUDY OBJECTIVES
- obtain the PK parameters of LPV/rtv and APV/rtv alone and in combination (700 mg GW433908 + 400mg/100mg LPV/rtv) in HIV-infected subjects
- assess whether LPV and/or APV AUC12h and C12h is significantly lower when given in combination than when given alone
STUDY DESIGN
- subjects randomized to:
A) LPV 400 mg/rtv 100 mg BID
B) 908 700 mg + RTV 100 mg BID
C) LPV 400 mg/rtv 100mg BID + 908 700 mg BID
- all received tenofovir 300 mg QD + 1 or 2 nRTIs, based on a VirtualPhenotype
- could have had prior experience with either APV (or GW433908) or LPV but not both
- randomization occurred in a 1:1:2 ratio for subjects naïve to both APV (or GW433908) and LPV
- subjects with experience to one of the PIs were randomized 1:1 to either single PK-enhanced arm to which they were naïve or the double PK-enhanced PI arm.
Substudy Design
- steady-state PK sampling performed at week 2 (14-28 days after starting study treatment)
- 8 blood samples collected over 12 hours (t = 0, 1, 2, 4, 6, 8, 10, 12hr)
- sampling followed an observed, simultaneous administration of the first morning dose of all ARVs
- drug concentration and PK analyses to be performed in real-time
METHODS
Drug Concentration Analyses
- determined using a validated LC/UV assay (at the University of Alabama at Birmingham Pharmacology Support Laboratory) - LLOQ for APV, LPV, and RTV : 50ng/mL PK Analyses
- noncompartmental analyses performed using WinNonlin software (Pharsight Corporation, version 4.0.1).
- AUC from 0-12h calculated using the linear/log trapezoidal rule Statistical Analyses:
- comparisons on the logarithmic scale
- mean AUC12h or C12h were compared with a one-sided t-test, assuming unequal variances
- stopping guidelines specified a Peto stopping boundary (p<0.001) for comparison between treatment arms an independent review committee performed the interim review
RESULTS
- 44 subjects enrolled between 10/2002 and 6/2003
- 8 subjects had outstanding PK data
- 3 subjects (1 in each arm) had unusable PK data
- data on 33 subjects used in interim review
- comparisons were restricted to subjects who were eligible to be randomized to both arms that were compared (n=15 for each comparison in the LPV/rtv+ 908 arm)
|
|
| |
| |
 |
|
| |
| |
 |
|
| |
| |
 |
|
| |
| |
 |
|
| |
| |
Discussion by authors
Amprenavir and Lopinavir AUC12h and C12h are reduced when GW433908 and LPV/rtv are combined. There are no obvious RTV- mediated interaction: RTV concentrations comparable between groups.
There is no apparent TDF-mediated interaction: APV and RTV concentrations in the 908/rtv arm, and LPV and RTV concentrations in the LPV/rtv arm are similar to published data
Comparison of PK parameters suggests this occurs during absorption - since APV, LPV, and RTV all have CYP3A4 (and possibly drug transporter) inhibition and induction effects, other mechanisms cannot be ruled out
GSK data (on file) suggest this interaction cannot be overcome by › RTV, 908, or LPV/rtv doses (although LPV concentrations increased).
UNC-Chapel Hill data (on file) suggest this interaction cannot be overcome by separating the 908 and LPV/rtv doses by 4-12 hours and adding more RTV (although LPV concentrations increased) (A. Kashuba, unpublished data).
This study design allowed rapid identification of this drug-drug interaction in real-time, and provided information that will impact on the choice of PIs in patients with HIV who have virologic failure.
|
|
| |
|
 |
 |
|
|