| |
Enfuvirtide (T-20): A Novel Human Immunodeficiency Virus Type 1 Fusion Inhibitor
|
| |
| |
Enfuvirtide (T-20): A Novel Human Immunodeficiency Virus Type 1 Fusion Inhibitor
Clinical Infectious Diseases 2003;37:1102-1106 Reviews of Anti-Infective AgentsAG ENTS Joseph S. Cervia and Miriam A. Smith
Department of Internal Medicine, Division of Infectious Disease, Long Island Jewish Medical Center, Long Island Campus for the Albert Einstein College of Medicine, New Hyde Park, New York
ABSTRACT
The development of highly active antiretroviral therapy has improved life expectancy and reduced progression to acquired immunodeficiency syndrome in human immunodeficiency virus (HIV)infected patients. However, resistance to currently available classes of antiretroviral drugs has become a problem, limiting the options for patients with advanced disease who have been heavily treated. Enfuvirtide (T-20; ENF), a synthetic peptide, is the first of a new class of antiretrovirals that block entry of virus into host cells. ENF interferes with conformational changes required for membrane fusion and injection of virus into the host cell. Optimal treatment of HIV infection will likely require combinations of drugs that target novel stages of HIV type 1 entry and replication.
The development of HAART has improved life expectancy and reduced progression to AIDS in patients who are HIV infected. Of concern is the emergence of resistance to currently available classes of antiretroviral drugs and toxicities associated with these agents. Investigation continues to develop strategies to effectively treat HIV-infected patients. One of the strategies has been to identify previously unexplored areas of HIV-1 action, which has opened up the field of antiretroviral therapy to include agents that block entry of virus into host cells.
By 1993, it had been recognized that a synthetic peptide directed against the gp41 transmembrane portion of the HIV envelope had remarkable in vitro antiretroviral activity. Initially known as "DP-178," it was later renamed "T-20" and more recently renamed "enfuvirtide" (ENF) by its clinical developers at Trimeris and Roche. ENF represents the first of a new family of antiretrovirals that inhibit entry of HIV into host cells.
BACKGROUND
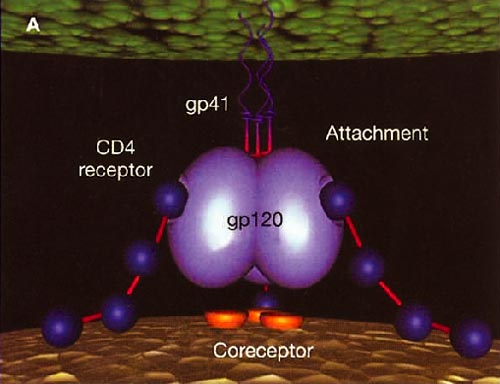
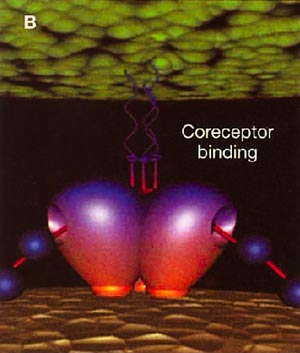
Infection with HIV-1 occurs after a series of events involving attachment of virus and subsequent entry of virus into host cells. The process of virus entry begins with an interaction of the external viral envelope glycoprotein, gp120, with the host cell CD4+ and chemokine receptor sites. A structural change probably occurs thereafter within gp120, allowing further interaction between gp120 and the major HIV coreceptors, CC chemokine receptor 5 (CCR5) and CXC chemokine receptor 4 (CXCR4). These changes precede conformational changes in the HIV envelope glycoprotein 41 (gp41), which plays a pivotal role in the fusion of viral and target cell membranes.
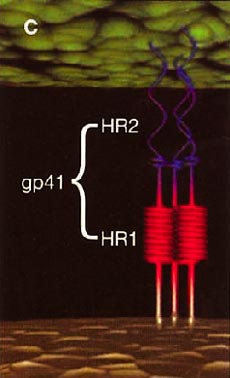
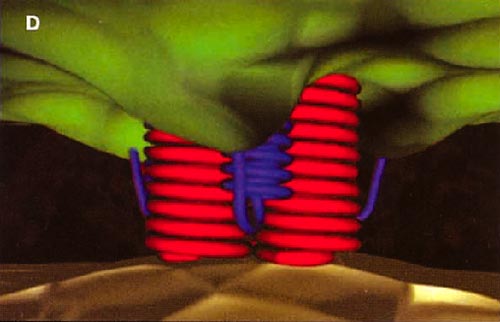
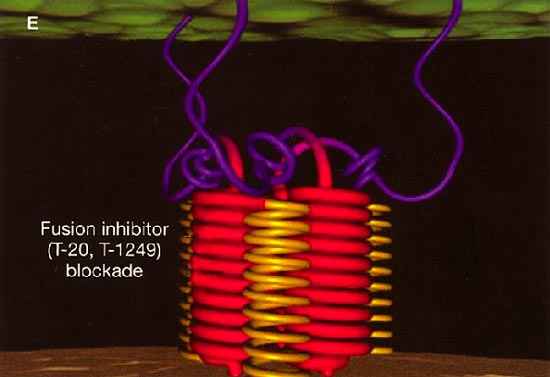
HIV cell entry mechanism of action of enfuvirtide. A, Attachment. B, Coreceptor binding. C, HIV insertion. D, Fusion. E, Inhibition of HIV fusion and infection. HR, helical region.
|
| |
| |
 |
|
| |
| |
 |
|
| |
| |
 |
|
| |
| |
 |
|
| |
| |
 |
|
| |
| |
IN VITRO ACTIVITY
The extracellular domain of gp41 contains a fusion peptide (FP) and 2 helical regions (HRs), HR1 and HR2. The FP region is made up of hydrophobic, glycine-rich residues essential for initiation of penetration into target cell membranes [1, 3, 4]. When fusion occurs, FP inserts into the target cell membrane, and HR1 and HR2 alter their conformation to form a 6-helix structure. The process results in the formation of a fusion pore through which the HIV capsid passes into the CD4+ cell [1, 3].
ENF is a synthetic peptide corresponding to the 36-aa sequence of the HR2 domain in gp41. ENF binds to the HR1 domain in the gp41 subunit of the viral envelope protein, which prevents the formation of the 6-helix structure and interferes with the conformational changes required for membrane fusion. ENF, in effect, binds to a structural intermediate of the fusion process, which impedes the transition of gp41 into a fusion-active state [1, 3, 5].
Ketas et al. [6] studied fusion inhibition using diverse primary cell types that represent major targets both for direct infection and for trans infection of target cells by virus-bound dendritic cells. Their study concluded that despite minor cell typedependent differences in potency, ENF provided effective inhibition on each cell type and that trans infection was vulnerable to ENF inhibition. Sensitivity of HIV-1 to fusion inhibition is influenced by coreceptor specificity, mutations in the HR1 of gp41, alterations in the V3 loop in gp120, and other determinants in the envelope outside the HR1 domain [1, 3, 7, 8].
Derdeyn et al. [7] analyzed 14 ENF-naive primary HIV isolates for sensitivity to ENF and found that the mean IC50 for isolates that used CCR5 for entry (R5 viruses) was greater than that for isolates that used CXCR4 (X4 viruses) (P = .0055). Additional study involving NL4.3-based chimeras containing combinations of envelope sequences of R5 and X4 viruses revealed that determinants of coreceptor specificity contained within the gp120 V3 loop modulate susceptibility to ENF. The IC50 for chimeric envelope viruses containing R5 V3 sequences was higher than that for viruses containing X4 V3 sequences.
Changes in coreceptor affinity and expression could significantly alter ENF susceptibility [3, 9]. Because ENF susceptibility is influenced by CCR5 expression levels, it is postulated that individuals with lower levels of CCR5 will generally respond better to ENF than will individuals with higher levels. The full extent to which CCR5 expression influences viral tropism and pathogenicity, compared with CXCR4 expression, is unclear. The relationship between coreceptor expression levels and the affinity with which an individual's predominant virus type binds to coreceptors may have a significant impact in vivo on the effectiveness of entry inhibitors [3].
It has been postulated that ENF targets viral envelope only during a kinetic window that is opened by CD4+ binding and closed by coreceptor attachment. Reeves et al. [3] attempted to identify viral and cellular determinants of ENF sensitivity. The group undertook a series of experiments that used receptor-binding assays and measured fusion kinetics. They were able to determine that, for various viral envelopes tested, differences in binding to ENF correlated with alterations in viral envelope/coreceptor affinity, which correlated with the kinetics of the fusion process. Viral envelope with high-affinity binding to coreceptor fused more quickly than did viral envelope with lower affinity. Enhanced affinity is thought to reduce the kinetic window during which viral envelope is sensitive to ENF. Higher levels of coreceptor resulted in more-rapid membrane fusion and increased resistance to ENF.
Development of inhibitors that target distinct stages of HIV-1 entry is under investigation [10, 11]. In vitro synergy has been observed between ENF and AMD-3100, a small-molecule inhibitor of HIV-1 attachment to CXCR4 [12]. In vitro synergy was also seen with ENF and SCH-C, a small-molecule antagonist of HIV coreceptor CCR5. This synergy has been noted in R5 HIV isolates that harbored resistance mutations to reverse-transcriptase and protease inhibitors [13]. Similarly, PRO 542, which neutralizes HIV by blocking its attachment to CD4+ cells, has shown synergistic inhibition of virus-cell fusion with ENF. This synergy was observed for phenotypically diverse viruses and a broad range of drug concentrations [14]. The enhanced activity of the PRO 542/ENF combination suggests that the multistep nature of HIV entry leaves the virus vulnerable to combinations of entry inhibitors, which provides a rationale for evaluating such combinations in clinical trials.
HIV-1 subtypes are geographically distributed. Subtype B is most common in North America and Europe, whereas non-B subtypes are most prevalent worldwide. However, most of the investigations of drug susceptibility have involved HIV-1 subtype B [5]. The occurrence of drug resistanceassociated mutations in the target genes of HIV-1 isolates recovered from treatment-naive patients has been reported and raises the question of whether the sequences in the HR1 domain of gp41 in different HIV subtypes contain mutations associated with ENF resistance. Xu et al. [5] and Hanna et al. [15] looked for resistance mutations in plasma samples obtained from subtype B and non-B HIV-1infected, ENF-naive patients, respectively. Both groups found that the GIV motif within the HR1 domain is highly conserved and that natural variants rarely occur. Primary resistance to ENF is rare, which provides further support for treatment of HIV infection with entry inhibitors.
CLINICAL PHARMACOLOGY
Intermittent injections of ENF have been found to be pharmacokinetically superior to continuous infusions and have been associated with fewer difficulties. In a 28-day, randomized, dose-comparison study of ENF involving 78 HIV-infected adults, it was found that plasma pharmacokinetics and antiviral responses were more consistent for subcutaneous injection than for continuous subcutaneous infusion because of technical difficulties experienced with the latter. Injection site reactions were common but generally mild [16].
CLINICAL TRIALS
In one study of 14 days of ENF monotherapy involving 16 HIV-infected adults, there appeared to be significant dose-related decreases in the plasma HIV RNA level. All subjects receiving 100 mg twice per day had decreases in the virus load to <500 copies/mL, as determined by a bDNA assay [17].
The largest phase 3 studies to date evaluating the efficacy of ENF were the T-20 versus Optimized Regimen Only (TORO) 1 trial (which enrolled 491 patients at 49 sites in North America and Brazil) and the TORO 2 trial (which enrolled 504 patients at 64 sites in Europe and Australia). Similar in design, these studies randomized patients who had prior treatment experience with 3 classes of antiretrovirals and HIV RNA levels of >5000 copies/mL to receive either an optimized background (OB) regimen of 35 antiretrovirals (selected on the basis of history and baseline genotypic and phenotypic resistance) alone or the OB regimen in combination with ENF (90 mg sc b.i.d.). Patients in both TORO 1 and TORO 2 were heavily treatment experienced; they had received an average of 12 prior antiretroviral drugs. More than 80% of subjects in both trials had virus with 5 primary mutations associated with resistance to the 3 antiretroviral classes. An average of 4 antiretrovirals were used in the OB regimens. At 24-week analysis, virus loads had decreased to a significantly greater degree in response to ENF plus OB, compared with OB alone (a mean difference of 0.934 log copies/mL in TORO 1 and 0.78 log copies/mL in TORO 2; P < .0001 for both). The magnitude of antiviral response in subjects treated with ENF was greatest in those with genotypic and phenotypic scores that indicated susceptibility to 2 other drugs with which the subject was treated. The percentages of subjects treated with ENF who achieved virus loads of <400 copies/mL were 31.7% and 28.4% in TORO 1 and 2, respectively, and the percentages of subjects who achieved virus loads of <50 copies/mL were 19.6% and 12.2%. Higher rates of pneumonia were noted among subjects receiving ENF than among those in the control groups. Injection site reactions were experienced by 98% of patients; however, only 3% discontinued therapy because of these reactions [18, 19].
The safety and efficacy of ENF were also assessed in a Pediatric AIDS Clinical Trials Group protocol, which enrolled 14 children aged 412 years with a median baseline plasma virus load of 26,866 copies/mL and a median CD4+ cell count of 523 cells/mm3. ENF was administered twice per day by subcutaneous injection at doses of 30 or 60 mg/m2 of body surface area. For 7 days, ENF was added to each patient's baseline antiretroviral regimen. At day 7, each subject's background antiretroviral therapy was changed to a regimen that was predicted to be virologically active while ENF was continued. Eleven of the 14 patients achieved a 0.7-log reduction in the HIV RNA level by day 7. By week 24, 10 subjects achieved virologic suppression of 1 log, 6 had virus loads of <400 copies/mL, and 3 had virus loads of <50 copies/mL [20].
TOXICITY AND TOLERABILITY
In a study designed to investigate patient satisfaction with long-term administration of ENF and the impact on activities of daily living of such treatment, patients' opinions were assessed by 2 questionnaires completed at baseline and week 48. A majority of the 70 patients who were receiving ENF with an average of 5 oral antiretrovirals agreed that subcutaneous injections of ENF had not affected the activities of daily living. Ninety-eight percent of patients stated that they would choose to continue receiving treatment with ENF if it were medically indicated [21].
ENF injections were well tolerated in the Pediatric AIDS Clinical Trials Group protocol investigating ENF. Although 1 child discontinued receiving the drug because of an aversion to injections, none of the 11 children who experienced injection site reactions discontinued the ENF regimen [20].
RESISTANCE
When added to failing antiretroviral regimens, the impact of ENF on virus load reduction appears to be transient, suggesting the development of resistance [22]. Poveda et al. [8] analyzed changes in the gp41 envelope regions in clinical samples obtained from heavily pretreated patients who also received ENF and developed virologic failure. All 4 patients had a rapid decrease in virus load within 1 month after starting therapy with ENF. However, all patients subsequently experienced viral rebound 23 months later. Interestingly, there appeared to be a virus-immunologic disconnect, in that all of the patients remained clinically asymptomatic and without opportunistic infection. The investigators found that the HR1 and HR2 domains were highly conserved throughout therapy with ENF, but they postulated that other envelope regions may be involved in the development of ENF resistance.
Antiretroviral resistance was analyzed for patients enrolled in the initial phase 1 clinical trial of ENF. The mean IC50s for the mutants G36D and V38A were significantly elevated (9.1-fold and 45-fold, respectively), compared with those for wild-type virus. In addition, the V38M mutation resulted in an 8-fold increase in the IC50, whereas the I37V mutation resulted in a 3.2-fold increase relative to that of wild-type virus [23].
SUMMARY
Inhibition of HIV entry into host cells provides a novel approach to the treatment of HIV infection. ENF represents the first of this new class of antiretrovirals to have been released. Sensitivity of HIV-1 to fusion inhibition is likely multifactorial, including coreceptor specificity, mutations in the HR1 of gp41, alterations in the V3 loop in gp120, and other determinants in the envelope outside the HR1 domain [1, 3, 7, 8]. In addition, the development of inhibitors that target distinct stages of HIV-1 entry is also under investigation [10, 11].
Data from clinical trials suggest that durable suppression of virus load with ENF depends on its use in combination with other antiretrovirals to which an individual patient's virus remains susceptible. ENF is a large peptide that is difficult and expensive to synthesize. Only small quantities of drug will be available initially, and the cost of therapy will be substantial (approximately $20,000 per patient per year). Sensitive as it is to proteolytic digestion, ENF lacks a bioavailable oral formulation. Repeated subcutaneous injection of this drug may reduce patients' adherence. The finding of virologic failure in heavily treated HIV-infected patients who received ENF raises concern regarding use of this agent alone or as the sole entry inhibitor in patients receiving HAART [8, 22].
These considerations will likely limit the use of ENF to those patients with advanced disease who have few remaining antiretroviral treatment options. Nevertheless, it will be important to use ENF while there are still other effective agents available for a given patient. Conserving ENF for deep-salvage patients with no other treatment options available appears to be a strategy doomed to fail. In addition, continuing ENF therapy in patients for whom it has failed, despite any potential value it may offer in terms of reduced viral replicative capacity, would be, at best, a very expensive strategy. At worst, continued ENF treatment for >48 weeks under such circumstances has been shown to contribute to the development of resistance to T-1249, a fusion inhibitor now in development that might otherwise serve as an element of salvage regimens for patients with ENF failure [24].
ENF has become the first US Food and Drug Administrationapproved HIV-1 fusion inhibitor. Inclusion of ENF in a HAART regimen provides an intriguing addition to HIV therapy. It is anticipated that small-molecule, nonpeptide HIV fusion inhibitors may be developed in the future that have mechanisms of action similar to that of ENF [2]. Optimal treatment of HIV infection will likely continue to require combinations of drugs that target novel stages of HIV-1 entry and replication.
References
1. Derdeyn CA, Decker JM, Sfakianos JN, et al. Sensitivity of human immunodeficiency virus type 1 to fusion inhibitors targeted to the gp 41 first heptad repeat involves distinct regions of gp41 and is consistently modulated by gp 120 interactions with the coreceptor. J Virol 2001; 75:860514. First citation in article | PubMed
2. Jiang S, Zhao Q, Debnath AK. Peptide and non-peptide HIV fusion inhibitors. Curr Pharm Des 2002; 8:56380. First citation in article | PubMed
3. Reeves JD, Gallo SA, Ahmad N, et al. Sensitivity of HIV-1 to entry inhibitors correlates with envelope/coreceptor affinity, receptor density, and fusion kinetics. Proc Natl Acad Sci USA 2002; 99:1624954. First citation in article | PubMed
4. Lambert DM, Barney S, Lambert AL, et al. Peptides from conserved regions of paramyxovirus fusion (F) proteins are potent inhibitors of viral fusion. Proc Natl Acad Sci USA 1996; 93:218691. First citation in article | PubMed
5. Xu L, Hue S, Taylor S, et al. Minimal variation in T-20 binding domain of different HIV-1 subtypes from antiretroviral-naïve and -experienced patients. AIDS 2002; 16:16846. First citation in article | PubMed
6. Ketas TJ, Frank I, Klasse PJ, et al. Human immunodeficiency virus type 1 attachment, coreceptor, and fusion inhibitors are active against both direct and trans infection of primary cells. J Virol 2003; 77:27627. First citation in article | PubMed
7. Derdeyn CA, Decker JM, Sfakianos JN, et al. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp 120. J Virol 2000; 74:835867. First citation in article | PubMed
8. Poveda E, Rodes B, Toro C, et al. Evolution of the gp 41 env region in HIV-infected patients receiving T-20, a fusion inhibitor. AIDS 2002; 16:195961. First citation in article | PubMed
9. Laurence J. T-20: a model for novel anti-HIV drugs in development. AIDS Reader 2002; 12:46970. First citation in article | PubMed
10. DeClercq E. New developments in anti-HIV chemotherapy. Biochem Biophys Acta 2002; 1587:25875. First citation in article | PubMed
11. DeClerq E. New anti-HIV agents and targets. Med Res Rev 2002; 22:53165. First citation in article | PubMed
12. Tremblay CL, Kollmann C, Giguel F, et al. Strong in vitro synergy between the fusion inhibitor T-20 and the CXCR4 blocker AMD-3100. J Acquir Immune Defic Syndr 2000; 25:99102. First citation in article | PubMed
13. Tremblay CL, Giguel F, Kollmann C, et al. Antihuman immunodeficiency virus interactions of SCH-C (SCH 351125), a CCR5 antagonist, with other antiretroviral agents in vitro. Antimicrob Agents Chemother 2002; 46:13369. First citation in article | PubMed
14. Nagashima KA, Thompson DAD, Rosenfield SI, et al. Human immunodeficiency virus type 1 entry inhibitors PRO 542 and T-20 are potentially synergistic in blocking virus-cell and cell-cell fusion. J Infect Dis 2001; 183:11215. First citation in article | Full Text | PubMed
15. Hanna SL, Yang C, Owen SM, Lal RB. Resistance mutation in HIV entry inhibitors. AIDS 2002; 16:16038. First citation in article | PubMed
16. Kilby JM, Lalezari JP, Eron JJ, et al. The safety, plasma pharmacokinetics, and antiviral activity of subcutaneous enfuvirtide (T-20), a peptide inhibitor of gp 41mediated virus fusion, in HIV-infected adults. AIDS Res Hum Retroviruses 2002; 18:68593. First citation in article | PubMed
17. Kilby JM, Hopkins S, Venetta TM, et al. Potent suppression of HIV-1 replication humans by T-20, a peptide inhibitor of gp 41mediated virus entry. Nat Med 1998; 4:12323. First citation in article | PubMed
18. Lalezari JP, Henry K, O'Hearn M, et al. Enfuvirtide, an HIV fusion inhibitor, for drug-resistant HIV infection in North and South America. N Engl J Med 2003; 348:217585. First citation in article | PubMed
19. Clotet B, Lazzarin A, Cooper D, et al. Enfuvirtide (T-20) in combination with an optimized background (OB) regimen vs. OB alone in patients with prior experience or resistance to each of the three classes of approved antiretrovirals in Europe and Australia (TORO 2) [poster LbOr19A]. In: Proceedings of the 14th International AIDS Conference (Barcelona). Stockholm: International AIDS Society, 2002. First citation in article
20. Church JA, Cunningham C, Hughes M, et al. Safety and antiretroviral activity of chronic subcutaneous administration of T-20 in human immunodeficiency virus 1infected children. Pediatr Infect Dis J 2002; 21:6539. First citation in article | PubMed
21. Cohen CJ, Dusek A, Green J, et al. Long-term treatment with subcutaneous T-20, a fusion inhibitor, in HIV-infected patients: patient satisfaction and impact on activities of daily living. AIDS Patient Care STDS 2002; 16:32735. First citation in article | PubMed
22. Chen RY, Kilby JM, Saag MS. Enfuvirtide. Expert Opin Investig Drugs 2002; 11:183743. First citation in article | PubMed
23. Wei X, Decker JM, Liu H, et al. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother 2002; 46:1896905. First citation in article | PubMed
24. Mirales GD, Lalezari J, Bellos N, et al. T-1249 demonstrates potent antiviral activity over 10-day dosing in most patients who have failed a regimen containing enfuvirtide (ENF): planned interim analysis of T-1249-102, a phase I/II study [abstract 141b]. In: Program and abstracts of the 10th Conference on Retroviruses and Opportunistic Infections (Boston). Alexandria, VA: Foundation for Retrovirology and Human Health, 2003. First citation in article
|
|
| |
| |
|
|
|