| |
Occult hepatitis B in HCV+
|
| |
| |
Lancet Infect Dis 2002; 2: 479—86
Michael Torbenson and David L Thomas
MT is at the Department of Pathology, Division of Gastrointestinal and Hepatic Pathology at the Johns Hopkins Hospital, Baltimore, MD, USA; and DLT is at the John Hopkins School of Medicine, Baltimore, MD, USA.
A search on the NATAP website will find a number of articles about occult HBV. Here is an editorial by Anna Lok:
EDITORIAL: Occult hepatitis B virus infection in patients with hepatocellular carcinoma: Innocent bystander, cofactor, or culprit? - (01/15/04) http://www.natap.org/2004/jan/011504_08.htm
Worldwide, chronic hepatitis B virus (HBV) infection is the primary cause of cirrhosis and hepatocellular carcinoma and is one of the ten leading causes of death. Traditionally, people with chronic HBV infection have been identified with blood tests for HBV antigens and antibodies. Recently, another group of patients with chronic HBV infection has been identified by sensitive, molecular testing for HBV DNA. Members of this group are often referred to as having occult hepatitis B because they are HBV-DNA positive, but hepatitis B surface antigen negative. Occult hepatitis B occurs in a number of clinical settings. In this review, we examine occult hepatitis B in people co-infected with hepatitis C, in whom occult hepatitis B has been associated with advanced fibrosis and diminished response to interferon. Although much more research is needed, existing reports justify a heightened awareness of the medical importance and means of testing for occult hepatitis B. Of particular interest, see the section below on the Clinical Relevance of Occult HBV.
Hepatitis B virus (HBV) infection is the primary cause of cirrhosis and hepatocellular cancer (HCC) and one of the major causes of death globally. HBV can be transmitted from a mother to her infant, between sexual partners, within families, and by direct exposure to contaminated blood—eg, through injection drug use. After HBV infection, several distinct outcomes have been defined, traditionally based on the results of serological tests for HBV antigens and the antibodies produced to them. Although these classifications correspond well with clinical outcomes, advances in molecular testing challenge some conventional understandings of HBV infection.
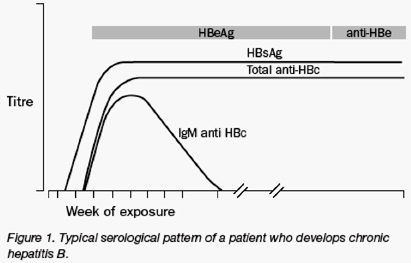
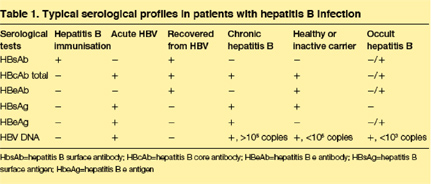
Many individuals seem to recover from acute HBV infection, since they do not have viral proteins or viral DNA in their blood. People who recover are generally not considered to be at risk for disease complications or for transmission of the infection. In other individuals, HBV infections persist (figure 1) and at least three distinct clinical states of viral persistence have been defined based on serological findings: chronic hepatitis B, the silent or "healthy" carrier, and occult hepatitis B (table 1). Chronic hepatitis B is defined clinically as the repeated detection of hepatitis B surface antigen (HBsAg) for 6 or more months after acute infection. Chronic hepatitis B is associated with high levels of HBV DNA, a high risk of transmission, liver inflammation, elevated liver enzymes, and the highest risk of cirrhosis and HCC. In addition, people with chronic hepatitis usually have high levels of the hepatitis B e antigen (HBeAg) in their serum samples, although some patients have mutations that prevent e protein expression (HBeAg-negative).
The healthy carrier state refers to people in whom HBsAg remains detectable in serum samples, but who have repeatedly normal (or minimally raised) liver enzymes and negative tests for HBeAg (table 1). These individuals are at low risk for progression to cirrhosis or HCC, and seem to be less infectious with lower levels of serum HBV DNA.
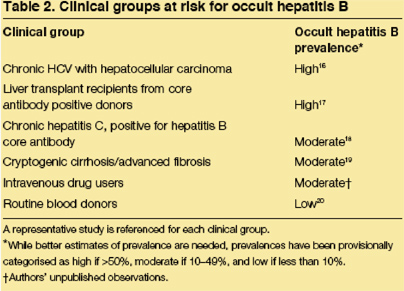
A final viral persistence group, occult hepatitis B, has recently been identified by sensitive PCR assays that may detect low levels of HBV DNA in the serum samples and/or liver of people who are HBsAg negative. With a typical chronic HBV infection, individuals are positive for both HBV DNA and HBsAg. In fact, the absence of surface antigenaemia has been used as a surrogate marker for the absence of DNA and active viral replication. Thus, the finding of HBV DNA in the serum of patients without HBsAg has been termed occult hepatitis B infection. In many instances, occult hepatitis B is associated with hepatitis B core antibody (HBcAb) and/or hepatitis B surface antibody (HBsAb). However, in a significant proportion of individuals, no HBcAb or HBsAb can be detected. Occult hepatitis B infection has been documented in a number of patient subgroups including those with HCV infection, HIV infection, hepatocellular carcinoma, and cryptogenic advanced liver fibrosis (table 2).
|
|
| |
| |
 |
|
| |
| |
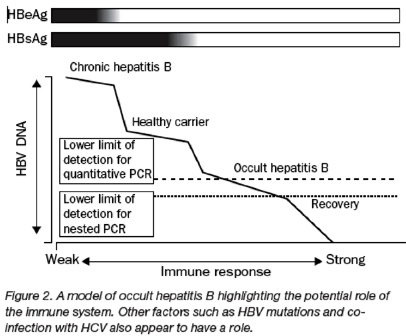
Patients with occult hepatitis B overlap with those who would previously have been classified as having recovered. In fact, the distinction between recovery and occult hepatitis B is likely to be somewhat arbitrary, since recovery does not necessarily imply eradication of infection in all cases, but includes the possibility of complete suppression in some cases by a broad and vigorous immune response (figure 2).
Accordingly, cases of "recurrent" HBV infection have been reported in people who previously recovered, but then became immunosuppressed. The most important clinical question is whether occult hepatitis B merely represents a marker of past infection, or whether HBV genome persistence contributes to liver disease.
In this review, we discuss laboratory testing issues, worldwide prevalence, clinical significance, and the biological basis for occult hepatitis B. We focus on occult hepatitis B in patients with chronic hepatitis C virus (HCV) infection, since the broader topic of occult hepatitis B has been recently reviewed. Occult hepatitis B has likewise been referred to by a variety of other names such as "inapparent HBV infection", "serologically silent hepatitis B", "silent hepatitis B", "surface antigen negative carriers", and "unrecognised hepatitis B infection", but the term occult hepatitis B will be used in this review.
|
|
| |
| |
 |
|
| |
| |
Laboratory testing for hepatitis B
Overview
Individuals with occult hepatitis B by definition are HBsAg negative. HBeAg is detectable only rarely. Individuals are variably positive for surface and core antibody, though about 20% are negative for all markers of past HBV infection aside from HBV DNA. Since viral DNA levels in occult hepatitis B are very low, the identification of occult hepatitis B is strongly dependent on both the sensitivity and specificity of the assay.
|
|
| |
| |
 |
|
| |
| |
Whereas this is generally true for all laboratory-based diagnoses, it is particularly relevant when investigating a disease that is identified only at a test's lower limit of detection, as is the case with occult hepatitis B. Nested PCR seems to be the most sensitive. Specificity can be demonstrated by direct sequencing, Southern blots or other DNA hybridization techniques, or by amplifying many gene targets.
|
|
| |
| |
 |
|
| |
| |
Antibody findings
Serological findings in patients with occult hepatitis B and HCV co-infection are summarised in table 3. Overall, 35% of people were HBsAb positive, 42% were HBcAb positive, and 22% were negative for both HBsAb and HBcAb. Prevalences differ in various studies and the actual prevalence in a specific population will vary depending on the hepatitis B risk factors. Overall, the prevalence of occult hepatitis B infection is higher in HBcAb positive than HBcAb negative patients. Furthermore, in one investigation patients with occult hepatitis B had higher titers of core, surface, and e antibodies than those without occult hepatitis B infection.
A number of studies have drawn attention to the increased prevalence of HBV antibodies in patients with chronic HCV infection, a rise that is not entirely explained by the prevalence of HBV in the general population. In particular, the "HBcAb-only" pattern has been associated with HCV infection. The HBcAb-only state refers to detection of HBcAb without the usual accompanying markers—HBsAg if the infection is chronic and HBsAb if the infection is resolved. For example, one investigation showed that 5% of patients with chronic HCV were coinfected with typical HBsAg positive chronic HBV infection, whereas 38% were positive for HBcAb only. Similarly, another study reported that 10% of HBsAb and HBcAb positive patients were anti-hepatitis C positive, whereas 49% of patients with HBcAb alone were anti-hepatitis C positive. Since HBV DNA can be detected in up to one-half of HBcAb-only patients, this disorder frequently represents an important subset of occult hepatitis B. In fact, the presence of HBcAb alone, in the absence of hepatitis B DNA testing, has been used in some studies as a marker of occult hepatitis B. Some instances of HBcAb-only serology seem to represent false positive results, even others represent recovery from a previous infection, since anamnestics response to HBV vaccines has been shown.
DNA testing
Because of the low DNA levels in occult hepatitis B, most studies have used nested PCR, in which two rounds of PCR amplification are done. At this time, no standardised PCR method for detecting occult hepatitis B exists and the sensitivities of the assays vary, ranging from 1--3 viral particles/mL of serum (nested PCR) to 600 copies/mL (single round PCR coupled with hybridisation). Others have reported sensitivity as DNA concentrations and have detected levels down to 1x10--6 pg to 1x10--1 ng. Little information is available on the optimal DNA extraction methods for detection of occult hepatitis B, though sample preparation is clearly important. Assay sensitivity, too, varies with the total amount of input DNA, the PCR primers, and assay conditions.
In most PCR assays, the X or S gene was amplified, with occasional targeting of the C gene or other regions of the hepatitis B genome. PCR of the S gene was the most sensitive in some but not all studies of serum, whereas the X gene was more sensitive in the liver. Hepatitis B DNA was generally not detectable on agarose gels after the first round of PCR, due to low starting template numbers. By contrast, HBV DNA was routinely detectable in the serum in patients with typical HBsAg positive HBV infection after a single round of PCR.
In sensitive PCR assays, there is a pronounced risk of false positive results from contamination or amplification of non-HBV-DNA targets. The specificity of the results is enhanced by sequencing the amplicon, which likewise checks for sample contamination, hybridisation with labelled DNA probes, and amplification of more than one genomic segment.
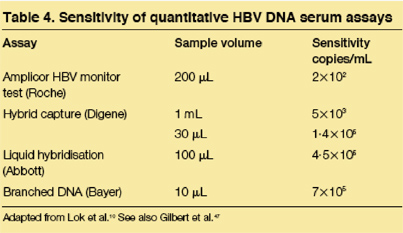
Quantitative hepatitis B DNA serum levels
A variety of testing methodologies are available to quantify HBV DNA (table 4), and it is virtually impossible to make direct comparisons due to the absence of standardization values. In occult hepatitis B, serum DNA levels are frequently below the limits of detection for many of the quantitative assays. In one investigation using the Amplicor HBV monitor test (reported sensitivity of 400 copies/mL; Roche Diagnostics, Raritan, NJ), HBV DNA was detectable in only eight of 15 patients positive for hepatitis B DNA on nested PCR. Those with detectable DNA had 400 copies/mL (n=3), 103 copies/mL (n=3), 104 copies/mL (n=1), or 106 copies/mL (n=1). By real-time PCR, hepatitis B DNA was quantifiable in 18 of 22 patients and ranged from 103 to 104 copies/mL. By dot blot assays, all cases of occult hepatitis B had less than 105 viral copies per mL. With the Digene assay in a study of non-HCV infected patients, the median HBV DNA concentration in occult hepatitis B was 2·2 pg/mL, range 0·69--47·8 pg/mL. By contrast, median DNA levels in HBsAg-positive controls were 350-fold higher at 774 pg/mL, range 0·7--2932 pg/mL. Patients with S gene mutations had
|
|
| |
| |
 |
|
| |
| |
higher serum hepatitis B DNA levels than patients without S gene mutations. In another investigation, HBV DNA in patients with occult hepatitis B was ten times lower than in HBsAg-positive patients by radioactive-labeled PCR and scintillation counting.
Prevalence of occult hepatitis B
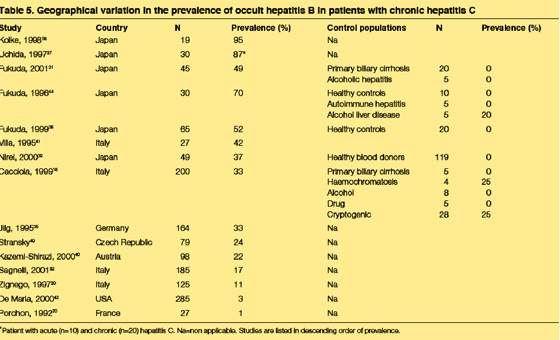
Geographical variability in the prevalence of occult hepatitis B has not been carefully studied. A single multi-nation investigation recorded the prevalence of occult hepatitis B in liver tissue to be 11% in Italy, 6·9% in Hong Kong, and 0% in the UK.48 Considering data from individual studies, there seems to be a high prevalence in Asia (table 5); (USA 3%). However, data from individual studies are difficult to compare since the study populations differ significantly in factors that probably change the prevalence of occult hepatitis B, such as the HBV risk factors in the population and the HBV DNA assay used. Higher rates of occult hepatitis B would clearly be expected in settings in which there were more people at risk for HBV infection (as indicated by HBV risk factors or positive antibody tests) and perhaps even higher rates in immunosuppressed populations (such as among HIV infected patients). Conversely, occult hepatitis B is rarely detected in control populations, such as among blood donors or people with non-viral types of chronic liver disease (table 5).
|
|
| |
| |
 |
|
| |
| |
Source of HBV DNA
Low levels of HBV replication have been detected in a number of extrahepatic tissues, including peripheral blood mononuclear cells, raising the possibility that serum HBV DNA in occult hepatitis B infection reflects non-hepatic replication. However, HBV DNA is routinely seen in the liver tissues of patients with serum DNA and can often be detected in liver tissue even when it is not detectable in serum. Furthermore, patients without HBV DNA in the liver do not have detectable HBV DNA in serum. The amount of HBV DNA in the liver tissue has been calculated at about one copy per 100 to 1000 cells in most cases.
Hepatic replication of HBV virus is further demonstrated by the hepatic expression of viral-specific proteins (core and surface) (figure 3), which are routinely detectable in a subset of patients with occult hepatitis B, though the number of positive cells is low. The detection of HBsAg in the liver by immunohistochemistry in patients in whom HBsAg is not detected in serum, probably indicates greater assay sensitivity, perhaps due to greater concentrations of surface antigen in the liver or the use of different antibodies to detect the antigens. One study showed that the same hepatocytes were often positive for both hepatitis C and hepatitis B proteins, though this remains to be confirmed by other investigators.
The association between the presence and amount of immunostaining for viral proteins in the liver and the rate of viral replication has not been carefully assessed. For example, it is unclear whether the failure to detect viral proteins by immunohistochemistry in some of the cases of occult hepatitis B indicates sampling variability alone or implies a lower replicative rate in those negative cases. Furthermore, interpretation of negative surface antigen immunostains is complicated by the observation that surface antigen mutations can markedly degrade the ability of commercial antibodies to detect surface antigen in the serum and the uncertainty of how such mutations affect immunostaining in the liver.
Although HBV DNA seems to be present in greater concentrations in the liver of patients with occult hepatitis B (compared with their serum samples), the HBV DNA in the serum does not appear to merely represent release of cytoplasmic or integrated DNA into the serum from normal hepatocyte turn-over, since the molecular forms in the liver and sera differ. Hepatitis B DNA in the liver is detectable as a closed circular form, whereas in serum, DNA is detected as an interrupted loop that is typical of HBV DNA in infectious virions.
Clinical relevance of occult hepatitis B
Overview
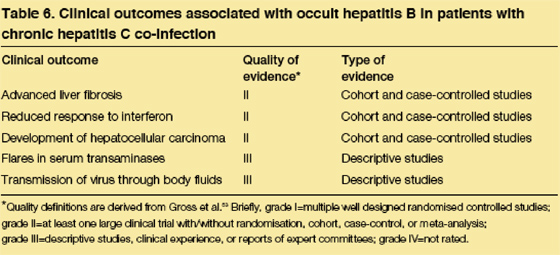
Although the presence of occult hepatitis B in chronic HCV infection is well established, the clinical relevance is still being assessed. Nevertheless, occult hepatitis B has been associated with advanced fibrosis/cirrhosis in cross-sectional studies and with poor interferon response in several treatment studies (table 6). Occult hepatitis B has likewise been reported in a high proportion of HCV-infected patients who develop hepatocellular carcinoma. Although only limited clinical and histological follow-up information is available, several studies have suggested that HBV replication accounts for many of the ALT flares in patients coinfected with HCV and occult hepatitis B.
|
|
| |
| |
 |
|
| |
| |
Serum enzyme levels and histological findings
The alanine aminotransferase (ALT) levels are usually mildly higher in occult hepatitis B and HCV co-infection, with means ranging from 39--158 IU/mL and seem to be either similar or mildly raised than in HCV mono-infection patients. ALT levels correlate well with the inflammatory activity on biopsy, which generally is mild. In a surprising contrast to the mild inflammation, most studies have demonstrated an increased prevalence of advanced fibrosis/cirrhosis in occult hepatitis B patients. For example, in a study of 200 patients, 33% of the patients with HCV and occult hepatitis B co-infection had cirrhosis versus 19% of HCV monoinfected patients. Although these studies are cross sectional, they imply that occult hepatitis B co-infection hastens the progression of fibrosis in patients with chronic HCV, though not all studies have supported an increased risk for fibrosis. Prospective studies are needed to clarify the relation between occult hepatitis B and fibrosis progression.
In chronic HCV infection, the presence of occult hepatitis B co-infection has been associated with a poor interferon-a response in many but not all studies. Similar findings are reported in individuals who are positive for isolated HBcAb. In one study, the end of treatment response to interferon-a was 57% in patients who were HBcAb negative, but only 32% in those patients who were HBcAb positive. Multivariate analysis confirmed that the association between exposure to hepatitis B and a reduced response rate to interferon-a was independent of other factors. The interferon-a response does not appear to be explained by associations with HCV genotypes, since most studies have not seen an association or have controlled for a higher prevalence of occult hepatitis B in patients with HCV genotype 1b. Although the potential mechanism for reduced interferon-a response remains unclear, one intriguing investigation has shown decreased intrahepatic expression of interferon receptor mRNA and protein in occult hepatitis B. Because antiviral efficacy is complex and multifactorial, additional studies are clearly needed to verify and measure the impact of occult hepatitis B in response to antiviral therapy.
The relation between occult hepatitis B and HCV RNA levels is unclear, with some studies reporting higher levels and others finding no difference. Furthermore, decreased HCV RNA levels were reported in patients with HBcAb compared to those without HBcAb, though most patients were HBV DNA negative.
Hepatocellular carcinoma
Epidemiological studies have strongly linked the development of HCC in individuals with chronic HCV infection to co-infection with HBV, including many patients who had apparently cleared their HB infection. Supporting this association, a high proportion of individuals with HCV infection who develop HCC have demonstrable HBV viral DNA and proteins in the neoplastic and adjacent non-neoplastic liver.
Furthermore, HBV DNA is present in the serum of up to 52% of HCV-infected individuals who are HBsAg negative and develop HCC. Rodent models of chronic HBV infection have confirmed this observation and have shown that animals are at an increased risk for HCC after HBV infection, even when they apparently cleared the infection by serological tests. HCCs in these rodent models contain HBV DNA. Finally, interferon-a therapy has been shown to reduce the risk for HCC mainly in HCV-infected patients who have no evidence for past exposure to HBV infection, whereas those who were HBcAb positive, had little risk reduction.
Hepatitis B contributes to tumour development through a number of mechanisms including disruption of cell signaling pathways, DNA repair mechanisms, and apoptotic pathways. As just one of many examples of dysregulated pathways, the TGF-beta signaling pathway has both fibrogenic and neoplastic associations. Furthermore, the HBV X gene product is a potent transactivator of a number of signalling pathways that probably contribute to HCC. In fact, HCCs arising in the setting of occult hepatitis B show clonal integration of HBV DNA in many but not all cases, and frequently express the viral X gene, which is often mutated.
Analysis of follow-up tissues
Several longitudinal studies have shown that HBV DNA detection can be intermittent. In one investigation, followup serum samples remained positive in two of eight cases, became negative in six of eight cases, and three previously negative cases became positive. Similarly, in a study with 40 follow-up specimens, the status of 27 cases remained the same, seven previously positive cases became negative, and six negative cases became positive. Follow-up studies have likewise shown that ALT flares can be associated with the detection of hepatitis B DNA in the serum (negative before and after flare) in patients with occult hepatitis B and HCV co-infection. In these cases, it seems that fluctuations in the levels of HBV DNA were clinically meaningful. Whether other instances merely represent testing variability is unknown, since the HBV DNA levels can be near the lower limits of detection for the assays.
Recommendations for occult hepatitis B testing
Because nested PCR tests for HBV DNA are not approved by the Food and Drug Administration, testing for occult hepatitis B has been done largely in research settings. There are no published guidelines as to which patients should be screened for occult hepatitis B. Some have recommended testing for occult hepatitis B in the HCV-infected patient when HCV RNA is cleared by treatment. Yet ALT levels remain elevated and are unexplained by other hepatic insults (eg, medications, fatty liver disease, etc). We agree with this recommendation and would add that clinicians should consider testing for occult hepatitis B in the following additional situations: (1) patients with risk factors for HBV infection in whom immunosuppression is expected (eg, cancer chemotherapy) because flares of occult HBV have been reported in this setting and can be treated successfully with antivirals; (2) patients with unexplained liver disease (either unexplained elevations in liver enzymes or cryptogenic cirrhosis); and (3) patients with isolated HBcAb in whom the presence of occult hepatitis B would affect clinical decisions (eg, solid organ donors and consideration of HBV vaccination).
Possible mechanisms
The molecular/immunological mechanisms underlying occult hepatitis B infection remain incompletely defined and are probably multifactorial. On the one hand, occult hepatitis B could merely show the greater sensitivity of the PCR-based testing used to detect HBV DNA, compared with the enzyme immunoassay tests used to detect serum antigens. In some cases, mutations in the hepatitis B surface gene could substantially reduce the sensitivity of the HBsAg assays by making the surface protein less immunoreactive with commercially available assays.
However, although assay sensitivity does not explain the basis for the low rate of HBV replication. One possibility is that occult hepatitis B represents one of a series of clinical stages determined by the host response to HBV infection (figure 2). For many years, studies in human beings and rodent models74 have reported that those who apparently clear a HBV infection continue to have low, but detectable, amounts of HBV DNA in the liver. In many cases, the immune system probably contains the hepatitis B infection, limiting viral replication to a minimum. This model fits the clinical observation that people can move between stages when there is a major abrogation of the immune response, such as that caused by receipt of cancer chemotherapy or HIV infection, or an enhancement of the immune response associated with interferon therapy. Transition between stages of infection could likewise be influenced by changes in viral factors such as mutations in the S or X gene. In this model, occult hepatitis B overlaps with HBV recovery according to the results of HBV DNA testing.
Other data suggest viral factors can be responsible for occult hepatitis B. Transmission of HBV from a patient with occult hepatitis B to a chimpanzee led to an infection with similarly low viraemia. Furthermore, in the transplant setting, transmission of occult hepatitis B from the donor allograft generally leads to a mild chronic infection, though patients frequently become HBsAg positive. Several possible mechanisms have been identified that might explain the low viral replication rate. Mutations in the X gene have been described and might reduce the ability of the X protein to transactive host cellular proteins essential in viral replication. Mutations in the core promoter region may likewise reduce viral replication.
Another possible explanation is that superinfection with HCV might directly contribute to many cases of occult hepatitis B. Hepatitis C infection reduces the expression of HBV proteins in the liver78 and the HCV core proteins suppress hepatitis B viral expression in cell cultures. Older people might likewise be predisposed to the loss of HBsAg, though studies to date have generally not reported large age differences between patients with typical hepatitis B and those with occult hepatitis B. Clearly, more work is needed to understand this recently recognised clinical disorder.
Conclusions
Occult hepatitis B is defined by the presence of HBV DNA in the serum or liver of people without HBsAg. Occult hepatitis B can be identified in a substantial subset of HCV-infected people, especially in those who are positive for HBcAb. Serum HBV DNA levels are characteristically low, but despite the low replication rate, HBV DNA detection can be associated with ALT flares and is associated with advanced fibrosis/cirrhosis. In addition, occult hepatitis B is associated with decreased interferon response in some HCV-infected patients. At this time, available data support testing for occult hepatitis B (ie, HBV DNA) in HCV-infected patients when the results will affect clinical management, including those with HBV risk factors in whom immunosuppression is expected, and people with unexplained liver disease. Additional studies with long-term clinical follow-up are needed to better understand the biological basis and clinical significance of occult hepatitis B in HCV-infected patients.
|
|
| |
| |
|
|
|