| |
NRTIs and Intracellular Activity and Toxicities for Women, Advanced HIV & Hepatitis
|
| |
| |
"The Cellular Pharmacology of Nucleoside- and Nucleotide-Analogue Reverse-Transcriptase Inhibitors and Its Relationship to Clinical Toxicities"
Clinical Infectious Diseases March 2004;38:743-753
Peter L. Anderson,1 Thomas N. Kakuda,2 and Kenneth A. Lichtenstein1
1University of Colorado Health Sciences Center, Denver, Colorado; and 2Roche Laboratories, Nutley, New Jersey
Comments From Jules Levin: these study regarding NRTI pharmacology and related toxicity are emerging at the same time as a heightened awareness regarding plasma levels of HIV PI & NNRTI drugs and their relationships to gender, ethnicity and hepatitis. These present study findings suggest the potential for added toxicities from NRTIs based on certain risk factors including female gender, advanced HIV disease and the presence of hepatitis along with HIV. But, the clinical implications of this preliminary data are not understood.
Selected Author Findings:
This review underscores the pressing need for more research in the area of cellular NRTI pharmacology as it relates to NRTI toxicity...to provide the safest, most informed, and rational use of NRTIs for patients, additional studies of cellular NRTI pharmacology will be needed...
HEPATITIS... One randomized study of IFN- with or without zalcitabine-zidovudine demonstrated significantly more peripheral neuropathies (P = .02) and cytopenias (P = .03) in the combination IFN- arm than in the zidovudine-zalcitabine only arm [26]. In a phase 12 study of didanosine alone followed by IFN-didanosine, excess clinical pancreatitis or elevations of serum amylase/lipase was observed during IFN-didanosine combination therapy (didanosine doses of <250 mg b.i.d. were associated with a pancreatitis incidence of 24%)...
(26. Fischl MA, Richman DD, Saag M, et al. Safety and antiviral activity of combination therapy with zidovudine, zalcitabine, and two doses of interferon-2a in patients with HIV. AIDS Clinical Trials Group Study 197. J Acquir Immune Defic Syndr Hum Retrovirol 1997; 16:24753)
... Thus far, only 1 study has addressed a possible relationship between NRTI phosphate concentrations in patients and NRTI toxicity: higher total zidovudine phosphate concentrations (mono-, di-, and tri-) in PBMCs were associated with reduced hemoglobin levels during zidovudine monotherapy, whereas no such relationships were found with plasma zidovudine concentrations...
...Most of the clinical manifestations of NRTI toxicities resemble mitochondrial diseases, and histologic evidence demonstrates abnormal mitochondria and/or mtDNA depletion in affected tissues. Studies show that NRTI triphosphates competitively inhibit mtDNA polymerase g in vitro...
WOMEN... In small descriptive studies of NRTI phosphorylation, one characteristic that correlates with higher intracellular PBMC concentrations is female sex. One study found median lamivudine triphosphate and zidovudine triphosphate concentrations that were 1.6- and 2.3-fold higher, respectively, in a group of 4 HIV-infected women than those in a group of 29 men who initiated zidovudine, lamivudine, and indinavir (P < .01), although zidovudine or lamivudine plasma concentrations did not differ according to sex [44]. A second study reported mean total zidovudine phosphate levels that were 2-fold higher in a group of 5 women than those in a group of 16 men (P = .004), and another report described carbovir triphosphate (the active triphosphate for abacavir) levels in a single woman that were 28-fold higher than the levels in a group of 4 men (66)...
(44. Anderson PL, Kakuda TN, Kawle S, Fletcher CV. Antiviral dynamics and sex differences of zidovudine and lamivudine triphosphate concentrations in HIV-infected individuals. AIDS 2003; 17:21596866. Harris M, Back D, Kewn S, Jutha S, Marina R, Montaner JS. Intracellular carbovir triphosphate levels in patients taking abacavir once a day. AIDS 2002; 16:11967)
... An increased number of cases of hyperlactatemia have been reported among patients treated with stavudine combined with IFN-ribavirin, which is counterintuitive on the basis of the in vitro ribavirin data. This raises concern about the possible pharmacological interaction between IFN and stavudine...
... HIV infection and advanced HIV disease are associated with highly elevated concentrations of proinflammation and cellular activation markers...
Although PBMCs are currently the only biological specimens in which NRTI phosphate concentrations can reasonably be measured, this should not discourage research because NRTI toxicities presumably occur at the mitochondrial level in tissues of nerves, the liver, fat, the pancreas, and so on. The data in this review were based on measurement of NRTI phosphate levels in PBMCs, and possible research avenues were identified on the basis of these data...this review underscores the pressing need for more research in the area of cellular NRTI pharmacology as it relates to NRTI toxicity, because these adverse events severely affect many patients... Drug-drug interaction studies involving HIV and hepatitis C virus treatments should also investigate whether IFN upregulates NRTI phosphorylation in patients... Studies are needed to determine whether NRTI triphosphate levels are higher in women than in men, which could also provide pharmacological insights into the predisposition to fluorouracil toxicity in women... [in] developing countries, these same issues may become more important, because women constitute the majority of the HIV-infected population of sub-Saharan Africa, and ARTs would presumably be rationed to patients with more-advanced disease on the basis of need...
ABSTRACT/Summary
Nucleoside- and nucleotide-analogue reverse-transcriptase inhibitors (NRTIs) require intracellular phosphorylation for antihuman immunodeficiency virus (HIV) activity and toxicity. Long-term toxicities associated with NRTIs may be related to overactivation of this process.
In vitro experiments have shown increased rates of NRTI and endogenous nucleoside phosphorylation to be associated with cellular activation. Patients with advanced HIV disease often have overexpression of cytokines, which corresponds to an elevated cellular activation state. These patients also have higher rates of NRTI phosphorylation and NRTI toxicity, suggesting an interaction between a proinflammatory biological state, NRTI phosphorylation, and toxicity.
Studies suggest that women may have higher rates of NRTI phosphorylation than do men, as well as increased risk for NRTI-induced toxicity. Future research is needed to understand the NRTI activation process and improve the long-term toxicity profile of NRTIs. Such research should include comparisons of NRTI phosphorylation according to sex and cellular activation state (i.e., elevated vs. low).
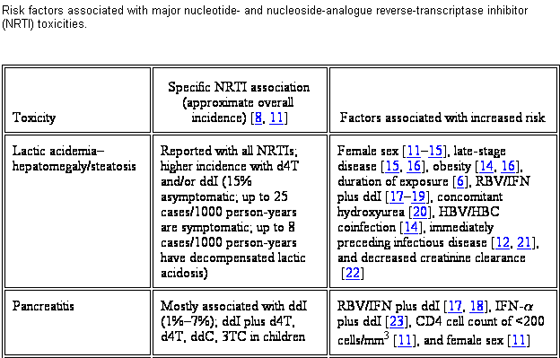
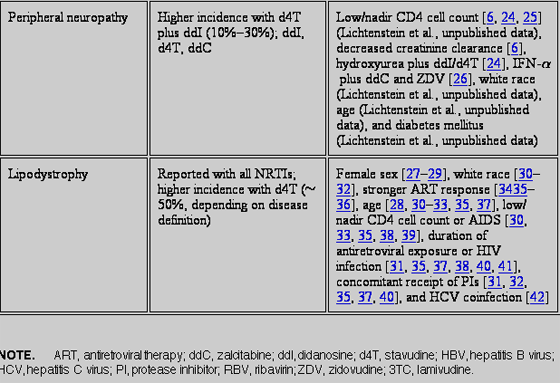
Treatment of HIV infection with combination antiretroviral therapy (ART) is a long-term undertaking. Although ART has been proven to reduce morbidity and mortality, drug toxicity issues have dampened enthusiasm for these accomplishments. Nucleoside- and nucleotide-analogue reverse-transcriptase inhibitors (NRTIs) play a central role in the treatment of HIV. Eight NRTIs are currently available: abacavir, didanosine, emtricitabine, lamivudine, stavudine, tenofovir disoproxil fumarate (nucelotide analogue), zalcitabine, and zidovudine. Several other NRTIs are in various stages of clinical development. Long-term toxicities attributed to NRTIs include hyperlactatemia and lactic acidosis, hepatomegaly with steatosis, peripheral neuropathy, myopathy and/or cardiomyopathy, ototoxicity, cytopenias, pancreatitis, and lipoatrophy. Cross-sectional studies identify recognized risk factors for the most common of these events. Limitations of the analyses such as study design, lack of a uniform toxicity definition (particularly in the case of lipodystrophy), and evaluations during ART compared with those during monotherapy or dual NRTI therapy should be considered when evaluating these risk factors.
RISK FACTORS ASSOCIATED WITH MAJOR NUCLEOTIDE & NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITOR (NRTI) TOXICITIES
|
|
| |
| |
 |
|
| |
| |
|
|
| |
| |
 |
|
| |
| |
Although NRTI toxicities are a major problem for patients infected with HIV, pharmacological research in this area is limited, in large part because measuring the active intracellular NRTI triphosphate concentrations in patients is difficult. The objective of this review is to address cellular NRTI activation as it relates to NRTI toxicity and to identify focus areas for future research. Information was included from epidemiological studies of NRTI toxicity, descriptive studies of NRTI phosphorylation in patients, and in vitro experiments.
NRTI PHARMACOLOGY
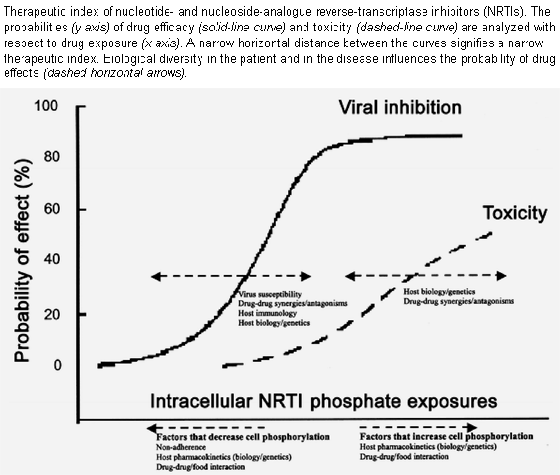
For all NRTIs, it is essential to consider drug exposures in terms of intracellular NRTI triphosphate concentrations, because these are the moieties that exert antiretroviral and toxic activities. This creates a distinctive therapeutic index for NRTIs, as depicted in figure 1, in which cellular NRTI activation can be one factor that influences antiretroviral effects and drug toxicity.
|
|
| |
| |
 |
|
| |
| |
The cellular activation of NRTIs produces at least 2 distinct sets of pharmacokinetic dispositions, one for the biologically inactive drug in plasma and the other for the active NRTI phosphate in cells. Although underlying relationships probably exist between plasma NRTI concentrations and intracellular NRTI phosphate concentrations, these relationships are currently unpredictable in patients. This is likely the result of rate-limiting or saturated-phosphorylation steps and the overall biological complexity of the system.
Most of the clinical manifestations of NRTI toxicities resemble mitochondrial diseases, and histologic evidence demonstrates abnormal mitochondria and/or mtDNA depletion in affected tissues. Studies show that NRTI triphosphates competitively inhibit mtDNA polymerase g in vitro. This, in turn, may decrease the number of mitochondrial respiratory chain proteins, inhibit aerobic respiration, induce oxidative stress, increase mutation in mtDNA, and result in mitochondrial and/or tissue failure. This mechanism of toxicity is a selectivity/specificity problem, and, therefore, toxicity will strongly depend on drug dose and concentration, as shown in figure 1. As such, elevations in NRTI triphosphate concentrations will significantly impact the mitochondrial toxicity of NRTIs.
To date, the cellular activation state for a given cell type has been determined to be the most influential factor for increased generation of intracellular NRTI phosphate concentrations, although this was elucidated in vitro. Cells treated with phytohemagglutinin or granulocyte-macrophage colony-stimulating factor (GM-CSF) generated 2- to >150-fold higher triphosphate concentrations of zalcitabine, lamivudine, stavudine, zidovudine, and didanosine (2,3-dideoxyadenosine triphosphate) than resting cells in vitro. Elevated cell activation results in high nucleic acid synthesis and an upregulation of kinases that phosphorylate NRTIs. It must be noted that zalcitabine, lamivudine, and didanosine are more active virologically in resting cells than in activated cells, which has been attributed to a more favorable ratio of NRTI triphosphate to endogenous nucleoside triphosphate (e.g., lamivudine triphosphate to 2-deoxycytidine-5 triphosphate ratio) in resting cells. Nevertheless, the actual concentrations of lamivudine, zalcitabine, and 2,3-dideoxyadenosine triphosphates were 24-fold higher in activated versus resting cells. It is not known whether the same cytoplasmic (whole cell) ratio is as relevant to NRTI toxicities in the mitochondrial compartment as it is to antiviral effects in the cytoplasmic/nuclear compartment.
The higher incidences of pancreatitis and peripheral neuropathy observed in patients receiving hydroxyureaa ribonucleotide reductase inhibitor were hypothesized to be caused by decreased endogenous deoxynucleotide pools in cytoplasm and, thus, a ratio favoring the NRTI triphosphates, particularly didanosine (2,3-dideoxyadenosine triphosphate). However, a study in patients treated with hydroxyurea could not detect changes in endogenous deoxynucleotide pools, including deoxyadenosine triphosphate pools. Hydroxyurea may also upregulate salvage pathways of nucleoside phosphorylation, and it arrests cells in the G1-S phase, which may upregulate generation of NRTI phosphates and could also explain increased toxicity. These multiple possibilities of hydroxyurea effects make it difficult to resolve the importance of the cytoplasmic ratio for NRTI toxicities in the mitochondrial compartment.
In the mitochondrial compartment, there are kinases that potentially phosphorylate NRTIs, but a nucleotide carrier protein may also shuttle nucleotides from cytoplasm into the mitochondrion. This nucleotide carrier has a stronger affinity for many NRTI phosphates than the corresponding endogenous nucleotides, which may influence the importance of the ratio with cytoplasmic endogenous nucleotides. As a possible illustration of the activity of this carrier, one in vitro study with zidovudine could not generate mitochondrial toxicity during exposure of isolated mitochondria to zidovudine. Instead, zidovudine was extremely toxic to mitochondria in activated whole cells, compared with resting whole cells, which may indicate significant NRTI phosphate shuttling into mitochondria to elicit the toxicity.
IN VIVO VERSUS IN VITRO NRTI TRIPHOSPHATE CONCENTRATIONS AND TOXICITY
Thus far, only 1 study has addressed a possible relationship between NRTI phosphate concentrations in patients and NRTI toxicity: higher total zidovudine phosphate concentrations (mono-, di-, and tri-) in PBMCs were associated with reduced hemoglobin levels during zidovudine monotherapy, whereas no such relationships were found with plasma zidovudine concentrations. Table 2 compares the typical NRTI triphosphate pharmacological data descriptively measured in patient's PBMCs, along with the NRTI triphosphate concentrations that inhibit mtDNA polymerase in vitro. Intracellular concentrations of 2,3-dideoxyadenosine, emtricitabine, lamivudine, and stavudine triphosphates reach levels near the in vitro binding affinity for polymerase . Table 2 also shows the in vitro therapeutic index of each NRTI; a low number indicates a narrow therapeutic index. Lamivudine values may need to be viewed in light of the exonuclease function of polymerase . Lamivudine monophosphate is excised 750-fold more rapidly than zalcitabine monophosphate, which presumably lessens the toxicity of lamivudine relative to that of zalcitabine. The other NRTIs are excised at rates between those of these 2 agents.
Clinically, zidovudine, didanosine, stavudine, and zalcitabine all underwent dose de-escalations during development to approximately one-half of the present-day dose, because the probability of toxicity was unacceptably high (mainly cytopenia and myopathy were associated with zidovudine, and pancreatitis and peripheral neuropathy were associated with didanosine, stavudine, and zalcitabine). This narrow margin of drug exposures that elicited the increased risk of clinical toxicity is indicative of a low therapeutic index, as depicted in figure 1. Zidovudine seems to have more clinical toxicity than would be predicted on the basis of the values in table 2. Zidovudine may exert activity on cellular and mitochondrial processes other than the inhibition of polymerase , such as inhibition of adenylate kinase, impairment of the mitochondrial ADP-ATP translocator, and uncoupling of the electron transport chain.
Of importance, the exact biochemical events that elicit clinical toxicities are not well understood, and specific pharmacological questions remain: Are concentrations of NRTI mono- or diphosphates important? Do changes in endogenous nucleotide pools in cytoplasm versus those in mitochondria contribute to toxicity? How do NRTI phosphates appear and disappear in the cytoplasmic versus mitochondrial cell compartments? Why do different tissues have different NRTI toxicity profiles?
INTRACELLULAR NRTI PHOSPHATE LEVELS AND CLINICAL TOXICITIES IN WOMEN
In small descriptive studies of NRTI phosphorylation, one characteristic that correlates with higher intracellular PBMC concentrations is female sex. One study found median lamivudine triphosphate and zidovudine triphosphate concentrations that were 1.6- and 2.3-fold higher, respectively, in a group of 4 HIV-infected women than those in a group of 29 men who initiated zidovudine, lamivudine, and indinavir (P < .01), although zidovudine or lamivudine plasma concentrations did not differ according to sex. A second study reported mean total zidovudine phosphate levels that were 2-fold higher in a group of 5 women than those in a group of 16 men (P = .004), and another report described carbovir triphosphate (the active triphosphate for abacavir) levels in a single woman that were 28-fold higher than the levels in a group of 4 men. These data provide pharmacological insight that is consistent with epidemiological studies that showed 4-fold more lipodystrophy during dual therapy with NRTIs, disproportionately high rates of lactic acidosis and pancreatitis, and stronger antiviral responses to NRTIs in women versus men, respectively. Of interest, women with cancer experienced 1.5-fold more severe toxicities with the use of the anticancer nucleoside analogue fluorouracil than did men, after adjustment for study, dose, body size, and age (P < .0001). Fluorouracil requires cellular activation for biological activity, using steps similar to those of zidovudine and stavudine.
INTRACELLULAR NRTI PHOSPHATE LEVELS IN PATIENTS WITH ADVANCED HIV DISEASE
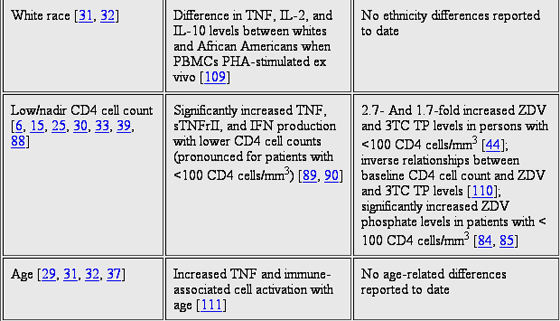
It has also been found that the presence and severity of HIV disease correlates with higher concentrations of intracellular NRTI phosphates in PBMCs. Among persons who received zidovudine monotherapy, the lowest intracellular zidovudine phosphate (mono-, di-, and tri-) concentrations were in healthy volunteers, followed by 5- and 12-fold higher concentrations in HIV-infected patients with CD4 cell counts of >100 and <100 cells/mm3, respectively. One small study also found high zidovudine monophosphate concentrations in patients with low CD4 cell counts, although the zidovudine triphosphate levels were lower.
A relationship between HIV disease severity and NRTI phosphorylation was also manifested in rates of NRTI phosphorylation in patients with advanced disease that were higher during the early stages of treatment relative to rates during later stages. In ART-naive patients initiating therapy with zidovudine, lamivudine, and indinavir, zidovudine triphosphate levels were 2.5-fold higher early in therapy (at week 2) in patients with a CD4 cell count of <100 cells/mm3 than they were in patients with higher CD4 cell counts. However, after 1 year of therapy, the zidovudine triphosphate levels in patients with advanced disease were reduced to the concentration range observed in patients with mild disease. In another zidovudine monotherapy study, the highest zidovudine phosphate concentrations were observed on day 1 of therapy; these concentrations decreased to 30% of this value when measured again 6 months later. These data suggest that the presence of HIV infection and advanced HIV disease status are associated with higher NRTI phosphate concentrations, particularly just after initiation of therapy.
This provides pharmacological insight that is consistent with the advanced disease risk factor for NRTI toxicity. For example, a CD4 cell count of <100 cells/mm3 was the strongest predictor of lipoatrophy for NRTI-treated subjects in the HIV Outpatient Study (HOPS) cohort. NRTI-associated peripheral neuropathy is 2-fold more common in persons with CD4 cell counts of <100 cells/mm3 than it is in persons with higher CD4 cell counts. Neuropathy risk in the HOPS cohort was highest in patients with CD4 cell counts of <100 cells/mm3 during the first months after initiating NRTI therapy, but it subsequently decreased over time. This finding is similar to the initially elevated and later decreased NRTI phosphate concentrations described in patients with advanced disease (Lichtenstein et al., unpublished data). In the dose de-escalation studies described above, patients with CD4 cell counts of <100 cells/mm3 experienced significantly increased NRTI toxicities.
Some investigators hypothesized that the genetic sequence of the polymerase g enzyme may vary among individuals, resulting in increased or decreased affinity for NRTI triphosphates. Specific sequence variants of the mtDNA polymerase ggene obtained from 14 patients with NRTI-associated lactic acidosis or peripheral neuropathy were compared with sequences from 45 patients without NRTI toxicity. The investigators found no correlations between variations in the DNA polymerase gene sequence and these NRTI-associated toxicities. Instead, low CD4 cell counts were found to be predictive of neuropathy.
RELATIONSHIP BETWEEN CELLULAR ACTIVATION AND NRTI PHOSPHORYLATION IN PATIENTS
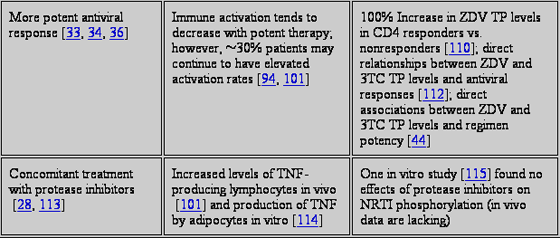
Just as cellular activation increases NRTI phosphorylation in vitro, an elevated state of cellular activation in patients with advanced disease may be a biological mechanism for increased NRTI phosphorylation in vivo. HIV infection and advanced HIV disease are associated with highly elevated concentrations of proinflammation and cellular activation markers, such as proinflammatory serum cytokines and molecules, IFN, TNF, and soluble TNF receptor type 2 (sTNFrII), and lymphocyte activation markers, such as CD38+ cells and HLA-DR+. For instance, compared with healthy volunteers, TNF and sTNFrII levels were 4-25-fold higher in patients with Centers for Disease Control and Prevention (CDC) class A and B HIV infection and 10-40-fold higher in patients with CDC class C HIV infection. As described in the previous section, these findings are similar to the apparent rates of NRTI phosphorylation in the same patient groups. In addition, treatment of advanced disease is known to significantly reduce the concentration of cell activation markers; this finding also corresponds to the reduction in the intracellular level of NRTI phosphate observed in patients with advanced disease after the disease had been controlled. Together, this points to a possible relationship between proinflammatory cellular activation and intracellular NRTI pharmacology and toxicity, but this possibility has not been adequately studied. One clinical study assessed total levels of zidovudine phosphate in PBMCs of patients initially treated with zidovudine alone followed by the cytokine GM-CSF plus zidovudine. GM-CSF is known to increase cell activation in patients, as shown by increased expression of HLA-DR+ on monocytes. An overall trend toward higher zidovudine phosphate levels was observed during combination GM-CSFzidovudine therapy in the entire cohort (P = .07), and higher doses of GM-CSF led to higher total zidovudine phosphate levels (P = .01).
TABLE 3
|
|
| |
| |
 |
|
| |
| |
|
|
| |
| |
 |
|
| |
| |
|
|
| |
| |
 |
|
| |
| |
|
|
| |
| |
 |
|
| |
| |
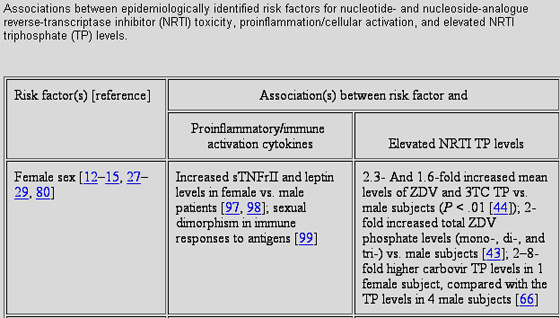
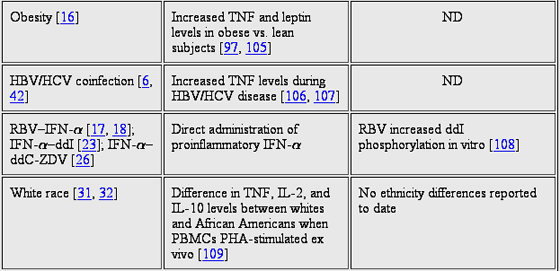
Table 3 presents relationships among proinflammatory cellular activation, NRTI triphosphate concentrations, and the epidemiological NRTI toxicity risk factors identified in table 1 including female sex, white race, age, hepatitis B and C coinfection, nadir CD4 cell count, and concomitant protease inhibitors. To provide some additional considerations, in animal models, NRTI toxicity could not be generated unless experimentally induced HIV infection was also present [116]. In phase 12 studies with fialuridine an investigational antihepatitis B virus nucleoside analogue that was urgently halted during the developmental phase because 7 of 15 patients died or required liver transplantation analysis of liver biopsy specimens obtained before initiation of treatment showed higher inflammation scores for the 7 patients who died or underwent liver transplantation than for patients who averted serious fialuridine toxicity [117, 118].
Coinciding with the possibility that advanced HIV disease and the corresponding cellular activation may increase NRTI triphosphate concentrations and thereby increase toxicity, inflammation may also cause biological harm that increases susceptibility to NRTI toxicities in an additive way [119, 120]. For illustration, ART-naive HIV-infected patients already show evidence of mtDNA depletion in PBMCs, compared with healthy volunteers, and, thus, NRTI-induced mtDNA depletion could be adding to preexisting mtDNA depletion [121]. In addition, the inflammation associated with HIV infection itself may harm bystander tissues, which occurs in HIV-associated peripheral neuropathy. Pathological analyses of nerve specimens obtained from these subjects indicate that activated macrophages infiltrate nerve tissue and may secrete destructive molecules [122]. Consistent with this finding, HIV-associated peripheral neuropathy occurs most frequently in the later stages of HIV infection. Patients with a history of nondrug-related neuropathy are at increased risk for NRTI-associated neuropathy [24, 122].
These observations identify a need to investigate how inflammation and cytokines affect NRTI cellular pharmacology and toxicity. One avenue for study should include determining how certain cytokines activate certain tissues and how this may stimulate high rates of NRTI phosphorylation in that tissue or cell type, which could contribute to the tissue selectivity of NRTI toxicities. For example, patients with ART-associated lipoatrophy and/or lipodystrophy express more TNF mRNA in subcutaneous fat and exhibit higher serum levels of sTNFrII and TNF than do control subjects [100, 101, 104, 123]. TNF exerts activity on fat [91, 124], and elevated TNF levels could specifically upregulate NRTI phosphorylation and/or susceptibility to NRTI toxicity in fat cells and tissue.
SPECIAL CONSIDERATIONS FOR CELLULAR NRTI ACTIVATION WITH CONCOMITANT ANTIHEPATITIS C VIRUS THERAPY
In the mid-1990s, studies evaluated the use of the proinflammatory cytokine IFN in combination with NRTIs to treat HIV infection. Increased NRTI toxicities were observed. One randomized study of IFN- with or without zalcitabine-zidovudine demonstrated significantly more peripheral neuropathies (P = .02) and cytopenias (P = .03) in the combination IFN- arm than in the zidovudine-zalcitabine only arm [26]. In a phase 12 study of didanosine alone followed by IFN-didanosine, excess clinical pancreatitis or elevations of serum amylase/lipase was observed during IFN-didanosine combination therapy (didanosine doses of 250 mg b.i.d. were associated with a pancreatitis incidence of 24%) [23].
Present concerns for drug-drug interactions between IFN-ribavirin and ARTs are focused on intracellular interactions between ribavirin and NRTIs, because in vitro studies showed that ribavirin enhanced conversion of didanosine to the active 2,3-dideoxyadenosine triphosphate [108, 125]. In vitro studies with ribavirin also showed that zidovudine and stavudine were less efficiently converted to their triphosphates [108, 126, 127]. Clinically, the risk of lactic acidosis and/or pancreatitis was 5% among patients coinfected with HIV/hepatitis C virus and cotreated with ART plus IFN-ribavirin, and the toxicity risk was significantly higher for didanosine recipients than for patients who received other NRTIs (OR, 18.3; P = .0002) [17]. However, the individual effects of ribavirin versus those of IFN on the increased risk of didanosine toxicity are not separable.
An increased number of cases of hyperlactatemia have been reported among patients treated with stavudine combined with IFN-ribavirin, which is counterintuitive on the basis of the in vitro ribavirin data [128]. This raises concern about the possible pharmacological interaction between IFN and stavudine. A small study of intracellular stavudine triphosphate concentrations in 15 patients before and after initiating IFN-ribavirin therapy found no statistical change in median peak concentrations 1 month after initiation of therapy, compared with baseline concentrations, although the range of peak concentrations increased (from a median of 26.4 and a range of 0.947.9 fmol per 106 PBMCs at baseline to a median of 14.7 and a range of 1.1148.3 fmol per 106 PBMCs at month 1) [71]. Future in vivo studies are needed to investigate intracellular interactions between NRTI phosphates and ribavirin, and such studies should also investigate the effects of the proinflammatory cytokine IFN.
CONCLUSIONS AND CLINICAL IMPLICATIONS
First, this review underscores the pressing need for more research in the area of cellular NRTI pharmacology as it relates to NRTI toxicity, because these adverse events severely affect many patients. Research activity may be stimulated by new mass spectrometry methods that could simplify the process for measuring NRTI phosphate concentrations in patients. Although PBMCs are currently the only biological specimens in which NRTI phosphate concentrations can reasonably be measured, this should not discourage research because NRTI toxicities presumably occur at the mitochondrial level in tissues of nerves, the liver, fat, the pancreas, and so on. The data in this review were based on measurement of NRTI phosphate levels in PBMCs, and possible research avenues were identified on the basis of these data.
Second, the observations in this review highlight specific research questions that warrant high priority, because the clinical implications are important. For example, the strategy of postponing administration of ART to ART-naive patients as long as possible to circumvent toxicitiesas is recommended by the current national guidelinesmay in fact predispose these patients with a state of elevated biological activation to increased NRTI toxicity. Investigations are needed to address a possible association between inflammatory cytokines and cellular NRTI pharmacology in patients. Drug-drug interaction studies involving HIV and hepatitis C virus treatments should also investigate whether IFN upregulates NRTI phosphorylation in patients. Studies are needed to determine whether NRTI triphosphate levels are higher in women than in men, which could also provide pharmacological insights into the predisposition to fluorouracil toxicity in women. If NRTIs can be widely supplied to developing countries, these same issues may become more important, because women constitute the majority of the HIV-infected population of sub-Saharan Africa, and ARTs would presumably be rationed to patients with more-advanced disease on the basis of need. Ultimately, to provide the safest, most informed, and rational use of NRTIs for patients, additional studies of cellular NRTI pharmacology will be needed.
References
-
US Public Health Service. Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. Available at: http://www.aidsinfo.nih.gov/guidelines/default_db2.asp?id=50. Accessed 12 January 2004.
-
Yeni PG, Hammer SM, Carpenter CC, et al. Antiretroviral treatment for adult HIV infection in 2002: updated recommendations of the International AIDS SocietyUSA Panel. JAMA 2002; 288:22235.
-
Powderly WG. Long-term exposure to lifelong therapies. J Acquir Immune Defic Syndr 2002; 29(Suppl 1):S2840.
-
Brinkman K, Kakuda TN. Mitochondrial toxicity of nucleoside analogue reverse transcriptase inhibitors: a looming obstacle for long-term antiretroviral therapy? Curr Opin Infect Dis 2000; 13:511.
-
Lenert LA, Feddersen M, Sturley A, Lee D. Adverse effects of medications and trade-offs between length of life and quality of life in human immunodeficiency virus infection. Am J Med 2002; 113:22932.
-
Moyle G. Clinical manifestations and management of antiretroviral nucleoside analogrelated mitochondrial toxicity. Clin Ther 2000; 22:91136; discussion 898.
-
Brinkman K, Smeitink JA, Romijn JA, Reiss P. Mitochondrial toxicity induced by nucleoside-analogue reverse-transcriptase inhibitors is a key factor in the pathogenesis of antiretroviral-therapyrelated lipodystrophy. Lancet 1999; 354:11125.
-
Carr A, Cooper DA. Adverse effects of antiretroviral therapy. Lancet 2000; 356:142330.
-
Dalakas MC. Peripheral neuropathy and antiretroviral drugs. J Peripher Nerv Syst 2001; 6:1420.
-
Chen D, Misra A, Garg A. Lipodystrophy in human immunodeficiency virusinfected patients. J Clin Endocrinol Metab 2002; 87:484556.
-
Dieterich DT. Long-term complications of nucleoside reverse transcriptase inhibitor therapy. AIDS Read 2003; 13:17684, 187. First citation in article | PubMed
-
Moyle GJ, Datta D, Mandalia S, Morlese J, Asboe D, Gazzard BG. Hyperlactataemia and lactic acidosis during antiretroviral therapy: relevance, reproducibility and possible risk factors. AIDS 2002; 16:13419. First citation in article | PubMed | CrossRef
-
Boxwell DE, Styrt BA. Lactic acidosis (LA) in patients receiving nucleoside reverse transcriptors (NRTIs) [abstract 1284]. In: Program and abstracts of the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy (San Francisco). Washington, DC: American Society for Microbiology, 1999:496. First citation in article
-
John M, Mallal S. Hyperlactatemia syndromes in people with HIV infection. Curr Opin Infect Dis 2002; 15:239. First citation in article | PubMed
-
Fortgang IS, Belitsos PC, Chaisson RE, Moore RD. Hepatomegaly and steatosis in HIV-infected patients receiving nucleoside analog antiretroviral therapy. Am J Gastroenterol 1995; 90:14336. First citation in article | PubMed
-
Coghlan ME, Sommadossi JP, Jhala NC, Many WJ, Saag MS, Johnson VA. Symptomatic lactic acidosis in hospitalized antiretroviral-treated patients with human immunodeficiency virus infection: a report of 12 cases. Clin Infect Dis 2001; 33:191421. First citation in article | Full Text | PubMed
-
Hor T, Deshayes J, Banisadr F, et al. Concomitant ddI/d4T and IFN (standard or pegylated)/ribavirin treatments may induce a high risk of mitochondrial toxicity in HIV/HCV infected patients (ANRS HCO2RIBAVIC Study) [abstract 1735]. In: Program and abstracts of the 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy (San Diego). Washington, DC: American Society for Microbiology, 2002. First citation in article
-
Lafeuillade A, Hittinger G, Chadapaud S. Increased mitochondrial toxicity with ribavirin in HIV/HCV coinfection. Lancet 2001; 357:2801. First citation in article | PubMed | CrossRef
-
Sauleda S, Juarez A, Esteban JI, et al. Interferon and ribavirin combination therapy for chronic hepatitis C in human immunodeficiency virusinfected patients with congenital coagulation disorders. Hepatology 2001; 34:103540. First citation in article | PubMed | CrossRef
-
Harris M, Tesiorowski A, Chan K, et al. Lactic acidosis complicating antiretroviral therapy: frequency and correlates [abstract 34]. Antivir Ther 2000; 5:31. First citation in article
-
Bonnet F, Bonarek M, Abridj A, et al. Severe lactic acidosis in HIV-infected patients treated with nucleosidic reverse transcriptase analogs: a report of 9 cases [in French]. Rev Med Interne 2003; 24:116. First citation in article | PubMed
-
Bonnet F, Bonarek M, Morlat P, et al. Risk factors for lactic acidosis in HIV-infected patients treated with nucleoside reverse-transcriptase inhibitors: a case-control study. Clin Infect Dis 2003; 36:13248. First citation in article | Full Text | PubMed
-
Kovacs JA, Bechtel C, Davey RT Jr, et al. Combination therapy with didanosine and interferon in human immunodeficiency virusinfected patients: results of a phase I/II trial. J Infect Dis 1996; 173:8408. First citation in article | PubMed
-
Moore RD, Wong WM, Keruly JC, McArthur JC. Incidence of neuropathy in HIV-infected patients on monotherapy versus those on combination therapy with didanosine, stavudine and hydroxyurea. AIDS 2000; 14:2738. First citation in article | PubMed | CrossRef
-
Chen X, Goudsmit J, van der Kuyl AC. Lack of correlation between length variation in the DNA polymerase gene CAG repeat and lactic acidosis or neuropathy during antiretroviral treatment. AIDS Res Hum Retroviruses 2002; 18:5314. First citation in article | PubMed | CrossRef
-
Fischl MA, Richman DD, Saag M, et al. Safety and antiviral activity of combination therapy with zidovudine, zalcitabine, and two doses of interferon-2a in patients with HIV. AIDS Clinical Trials Group Study 197. J Acquir Immune Defic Syndr Hum Retrovirol 1997; 16:24753. First citation in article | PubMed
-
Galli M, Ridolfo AL, Adorni F, et al. Body habitus changes and metabolic alterations in protease inhibitornaive HIV-1infected patients treated with two nucleoside reverse transcriptase inhibitors. J Acquir Immune Defic Syndr 2002; 29:2131. First citation in article | PubMed
-
Martinez E, Mocroft A, Garcia-Viejo MA, et al. Risk of lipodystrophy in HIV-1infected patients treated with protease inhibitors: a prospective cohort study. Lancet 2001; 357:5928. First citation in article | PubMed | CrossRef
-
Currier J, Carpenter C, Daar E, Kotler D, Wanke C. Identifying and managing morphologic complications of HIV and HAART. AIDS Read 2002; 12:1149, 1245. First citation in article | PubMed
-
Lichtenstein KA, Delaney KM, Armon C, et al. Incidence of and risk factors for lipoatrophy (abnormal fat loss) in ambulatory HIV-1infected patients. J Acquir Immune Defic Syndr 2003; 32:4856. First citation in article | PubMed
-
Mallal SA, John M, Moore CB, James IR, McKinnon EJ. Contribution of nucleoside analogue reverse transcriptase inhibitors to subcutaneous fat wasting in patients with HIV infection. AIDS 2000; 14:130916. First citation in article | PubMed | CrossRef
-
Lichtenstein KA, Ward DJ, Moorman AC, et al. Clinical assessment of HIV-associated lipodystrophy in an ambulatory population. AIDS 2001; 15:138998. First citation in article | PubMed | CrossRef
-
Chene G, Angelini E, Cotte L, et al. Role of long-term nucleoside-analogue therapy in lipodystrophy and metabolic disorders in human immunodeficiency virusinfected patients. Clin Infect Dis 2002; 34:64957. First citation in article | Full Text | PubMed
-
Cossarizza A, Mussini C, Vigano A. Mitochondria in the pathogenesis of lipodystrophy induced by anti-HIV antiretroviral drugs: actors or bystanders? Bioessays 2001; 23:107080. First citation in article | PubMed | CrossRef
-
Boufassa F, Dulioust A, Lascaux AS, et al. Lipodystrophy in 685 HIV-1treated patients: influence of antiretroviral treatment and immunovirological response. HIV Clin Trials 2001; 2:33945. First citation in article | PubMed
-
Wurtz R, Ceaser S. Adipose redistribution in human immunodeficiency virusseropositive patients: association with CD4 response. Clin Infect Dis 2000; 31:14978. First citation in article | Full Text | PubMed
-
Heath KV, Hogg RS, Chan KJ, et al. Lipodystrophy-associated morphological, cholesterol and triglyceride abnormalities in a population-based HIV/AIDS treatment database. AIDS 2001; 15:2319. First citation in article | PubMed | CrossRef
-
Thiebaut R, Daucourt V, Mercie P, et al. Lipodystrophy, metabolic disorders, and human immunodeficiency virus infection: Aquitaine cohort, France, 1999. Groupe d'Epidemiologie Clinique du Syndrome d'Immunodeficience Acquise en Aquitaine. Clin Infect Dis 2000; 31:14827. First citation in article | Full Text | PubMed
-
Mauss S, Corzillius M, Wolf E, et al. Risk factors for the HIV-associated lipodystrophy syndrome in a closed cohort of patients after 3 years of antiretroviral treatment. HIV Med 2002; 3:4955. First citation in article | PubMed | CrossRef
-
John M, Nolan D, Mallal S. Antiretroviral therapy and the lipodystrophy syndrome. Antivir Ther 2001; 6:920. First citation in article | PubMed
-
Rakotoambinina B, Medioni J, Rabian C, Jubault V, Jais JP, Viard JP. Lipodystrophic syndromes and hyperlipidemia in a cohort of HIV-1infected patients receiving triple combination antiretroviral therapy with a protease inhibitor. J Acquir Immune Defic Syndr 2001; 27:4439. First citation in article | PubMed
-
Zylberberg H, Nalpas B, Pol S, Brechot C, Viard JP. Is there a relationship between hepatitis C virus infection and antiretroviral-associated lipoatrophy? AIDS 2000; 14:2055. First citation in article | PubMed | CrossRef
-
Stretcher BN, Pesce AJ, Frame PT, Stein DS. Pharmacokinetics of zidovudine phosphorylation in peripheral blood mononuclear cells from patients infected with human immunodeficiency virus. Antimicrob Agents Chemother 1994; 38:15417. First citation in article | PubMed
-
Anderson PL, Kakuda TN, Kawle S, Fletcher CV. Antiviral dynamics and sex differences of zidovudine and lamivudine triphosphate concentrations in HIV-infected individuals. AIDS 2003; 17:215968. First citation in article | PubMed | CrossRef
-
Hoggard PG, Back DJ. Intracellular pharmacology of nucleoside analogues and protease inhibitors: role of transporter molecules. Curr Opin Infect Dis 2002; 15:38. First citation in article | PubMed
-
Stein DS, Moore KH. Phosphorylation of nucleoside analog antiretrovirals: a review for clinicians. Pharmacotherapy 2001; 21:1134. First citation in article | PubMed
-
Kakuda TN. Pharmacology of nucleoside and nucleotide reverse transcriptase inhibitorinduced mitochondrial toxicity. Clin Ther 2000; 22:685708. First citation in article | PubMed | CrossRef
-
Van Rompay AR, Johansson M, Karlsson A. Phosphorylation of nucleosides and nucleoside analogs by mammalian nucleoside monophosphate kinases. Pharmacol Ther 2000; 87:18998. First citation in article | PubMed | CrossRef
-
Dalakas MC, Semino-Mora C, Leon-Monzon M. Mitochondrial alterations with mitochondrial DNA depletion in the nerves of AIDS patients with peripheral neuropathy induced by 2,3-dideoxycytidine (ddC). Lab Invest 2001; 81:153744. First citation in article | PubMed
-
Dalakas MC, Illa I, Pezeshkpour GH, Laukaitis JP, Cohen B, Griffin JL. Mitochondrial myopathy caused by long-term zidovudine therapy. N Engl J Med 1990; 322:1098105. First citation in article | PubMed
-
Shikuma CM, Hu N, Milne C, et al. Mitochondrial DNA decrease in subcutaneous adipose tissue of HIV-infected individuals with peripheral lipoatrophy. AIDS 2001; 15:18019. First citation in article | PubMed | CrossRef
-
Johnson AA, Ray AS, Hanes J, et al. Toxicity of antiviral nucleoside analogs and the human mitochondrial DNA polymerase. J Biol Chem 2001; 276:4084757. First citation in article | PubMed | CrossRef
-
Martin JL, Brown CE, Matthews-Davis N, Reardon JE. Effects of antiviral nucleoside analogs on human DNA polymerases and mitochondrial DNA synthesis. Antimicrob Agents Chemother 1994; 38:27439. First citation in article | PubMed
-
Lewis W, Copeland WC, Day BJ. Mitochondrial DNA depletion, oxidative stress, and mutation: mechanisms of dysfunction from nucleoside reverse transcriptase inhibitors. Lab Invest 2001; 81:77790. First citation in article | PubMed
-
Robbins BL, Wilcox CK, Fridland A, Rodman J. Metabolism of tenofovir and didanosine in quiescent or stimulated human peripheral blood mononuclear cells. Pharmacotherapy 2003; 23:695701. First citation in article | PubMed
-
Perno CF, Cooney DA, Gao WY, et al. Effects of bone marrow stimulatory cytokines on human immunodeficiency virus replication and the antiviral activity of dideoxynucleosides in cultures of monocyte/macrophages. Blood 1992; 80:9951003. First citation in article | PubMed
-
Gao WY, Shirasaka T, Johns DG, Broder S, Mitsuya H. Differential phosphorylation of azidothymidine, dideoxycytidine, and dideoxyinosine in resting and activated peripheral blood mononuclear cells. J Clin Invest 1993; 91:232633. First citation in article | PubMed
-
Gao WY, Agbaria R, Driscoll JS, Mitsuya H. Divergent anti-human immunodeficiency virus activity and anabolic phosphorylation of 2,3-dideoxynucleoside analogs in resting and activated human cells. J Biol Chem 1994; 269:126338. First citation in article | PubMed
-
Robbins BL, Srinivas RV, Kim C, Bischofberger N, Fridland A. Antihuman immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), Bis(isopropyloxymethylcarbonyl)PMPA. Antimicrob Agents Chemother 1998; 42:6127. First citation in article | PubMed
-
Soler C, Garcia-Manteiga J, Valdes R, et al. Macrophages require different nucleoside transport systems for proliferation and activation. FASEB J 2001; 15:197988. First citation in article | PubMed | CrossRef
-
Hoggard PG, Kewn S, Maherbe A, et al. Time-dependent changes in HIV nucleoside analogue phosphorylation and the effect of hydroxyurea. AIDS 2002; 16:243946. First citation in article | PubMed | CrossRef
-
Kewn S, Hoggard PG, Sales SD, Johnson MA, Back DJ. The intracellular activation of lamivudine (3TC) and determination of 2-deoxycytidine-5-triphosphate (dCTP) pools in the presence and absence of various drugs in HepG2 cells. Br J Clin Pharmacol 2000; 50:597604. First citation in article | PubMed | CrossRef
-
Dolce V, Fiermonte G, Runswick MJ, Palmieri F, Walker JE. The human mitochondrial deoxynucleotide carrier and its role in the toxicity of nucleoside antivirals. Proc Natl Acad Sci U S A 2001; 98:22848. First citation in article | PubMed | CrossRef
-
Sales SD, Hoggard PG, Sunderland D, Khoo S, Hart CA, Back DJ. Zidovudine phosphorylation and mitochondrial toxicity in vitro. Toxicol Appl Pharmacol 2001; 177:548. First citation in article | PubMed | CrossRef
-
Stretcher BN, Pesce AJ, Frame PT, Greenberg KA, Stein DS. Correlates of zidovudine phosphorylation with markers of HIV disease progression and drug toxicity. AIDS 1994; 8:7639. First citation in article | PubMed
-
Harris M, Back D, Kewn S, Jutha S, Marina R, Montaner JS. Intracellular carbovir triphosphate levels in patients taking abacavir once a day. AIDS 2002; 16:11967. First citation in article | PubMed | CrossRef
-
Kewn S, Hoggard PG, Sales SD, et al. Development of enzymatic assays for quantification of intracellular lamivudine and carbovir triphosphate levels in peripheral blood mononuclear cells from human immunodeficiency virusinfected patients. Antimicrob Agents Chemother 2002; 46:13543. First citation in article | PubMed | CrossRef
-
Becher F, Landman R, Mboup S, et al. Monitoring of didanosine and stavudine intracellular triphosphorylated anabolite concentrations in HIV-infect patients. AIDS 2004; 18:1817. First citation in article
-
Rousseau FS, Kahn JO, Thompson M, et al. Prototype trial design for rapid dose selection of antiretroviral drugs: an example using emtricitabine (Coviracil). J Antimicrob Chemother 2001; 48:50713. First citation in article | PubMed | CrossRef
-
Rodriguez JF, Rodriguez JL, Santana J, Garcia H, Rosario O. Simultaneous quantitation of intracellular zidovudine and lamivudine triphosphates in human immunodeficiency virusinfected individuals. Antimicrob Agents Chemother 2000; 44:3097100. First citation in article | PubMed | CrossRef
-
Salmon-Ceron D, Lassalle R, Pruvost A, et al. Interferon-ribavirin in association with stavudine has no impact on plasma human immunodeficiency virus (HIV) type 1 level in patients coinfected with HIV and hepatitis C virus: a CORIST-ANRS HC1 trial. Clin Infect Dis 2003; 36:1295304. First citation in article | Full Text | PubMed
-
Pruvost A, Theodoro F, Hascoet S, et al. Development and validation of a direct LC/MS/MS assay for intracellular tenofovir-diphosphate in human PBMCs [abstract 837]. Antivir Ther 2003; 8(Suppl 1):S420. First citation in article
-
Traut TW. Physiological concentrations of purines and pyrimidines. Mol Cell Biochem 1994; 140:122. First citation in article | PubMed
-
Broder S. Pharmacodynamics of 2,3-dideoxycytidine: an inhibitor of human immunodeficiency virus. Am J Med 1990; 88:2S7S. First citation in article | PubMed
-
Yarchoan R, Pluda JM, Thomas RV, et al. Long-term toxicity/activity profile of 2,3-dideoxyinosine in AIDS or AIDS-related complex. Lancet 1990; 336:5269. First citation in article | PubMed | CrossRef
-
Fischl MA, Parker CB, Pettinelli C, et al. A randomized controlled trial of a reduced daily dose of zidovudine in patients with the acquired immunodeficiency syndrome. The AIDS Clinical Trials Group. N Engl J Med 1990; 323:100914. First citation in article | PubMed
-
Skowron G. Biologic effects and safety of stavudine: overview of phase I and II clinical trials. J Infect Dis 1995; 171(Suppl 2):S1137. First citation in article | PubMed
-
Browne MJ, Mayer KH, Chafee SB, et al. 2,3-Didehydro-3-deoxythymidine (d4T) in patients with AIDS or AIDS-related complex: a phase I trial. J Infect Dis 1993; 167:219. First citation in article | PubMed
-
Moyle G. Toxicity of antiretroviral nucleoside and nucleotide analogues: is mitochondrial toxicity the only mechanism? Drug Saf 2000; 23:46781. First citation in article | PubMed
-
Currier JS, Spino C, Grimes J, et al. Differences between women and men in adverse events and CD4+ responses to nucleoside analogue therapy for HIV infection. The AIDS Clinical Trials Group 175 Team. J Acquir Immune Defic Syndr 2000; 24:31624. First citation in article | PubMed
-
Sloan JA, Goldberg RM, Sargent DJ, et al. Women experience greater toxicity with fluorouracil-based chemotherapy for colorectal cancer. J Clin Oncol 2002; 20:14918. First citation in article | PubMed | CrossRef
-
Grem JL. Cancer chemotherapy principles and practice. Philadelphia: JB Lippincott, 1990. First citation in article
-
Barry MG, Khoo SH, Veal GJ, et al. The effect of zidovudine dose on the formation of intracellular phosphorylated metabolites. AIDS 1996; 10:13617. First citation in article | PubMed
-
Hoggard PG, Lloyd J, Khoo SH, et al. Zidovudine phosphorylation determined sequentially over 12 months in human immunodeficiency virusinfected patients with or without previous exposure to antiretroviral agents. Antimicrob Agents Chemother 2001; 45:97680. First citation in article | PubMed | CrossRef
-
Barry M, Wild M, Veal G, et al. Zidovudine phosphorylation in HIV-infected patients and seronegative volunteers. AIDS 1994; 8:F15. First citation in article | PubMed
-
Rodman JH, Robbins B, Flynn PM, Fridland A. A systemic and cellular model for zidovudine plasma concentrations and intracellular phosphorylation in patients. J Infect Dis 1996; 174:4909. First citation in article | PubMed
-
Anderson PL, Brundage RC, Weller D, Kawle SP, Bushman L, Fletcher CV. The pharmacokinetics of zidovudine-triphosphate (ZDV-TP) in HIV-infected adults [abstract 6.3]. In: Program and abstracts of the 3rd International Workshop on Clinical Pharmacology of HIV Therapy (Washington, DC). Utrecht, The Netherlands: Virology Education, 2002:6.3. First citation in article
-
Richman DD, Fischl MA, Grieco MH, et al. The toxicity of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex: a double-blind, placebo-controlled trial. N Engl J Med 1987; 317:1927. First citation in article | PubMed
-
Hestdal K, Aukrust P, Muller F, et al. Dysregulation of membrane-bound tumor necrosis factor- and tumor necrosis factor receptors on mononuclear cells in human immunodeficiency virus type 1 infection: low percentage of p75-tumor necrosis factor receptor positive cells in patients with advanced disease and high viral load. Blood 1997; 90:26709. First citation in article | PubMed
-
Godfried MH, van der Poll T, Jansen J, et al. Soluble receptors for tumour necrosis factor: a putative marker of disease progression in HIV infection. AIDS 1993; 7:336. First citation in article | PubMed
-
Finck BN, Johnson RW. Tumor necrosis factor- regulates secretion of the adipocyte-derived cytokine, leptin. Microsc Res Tech 2000; 50:20915. First citation in article | PubMed | CrossRef
-
Martin-Romero C, Santos-Alvarez J, Goberna R, Sanchez-Margalet V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol 2000; 199:1524. First citation in article | PubMed | CrossRef
-
Kestens L, Vanham G, Vereecken C, et al. Selective increase of activation antigens HLA-DR and CD38 on CD4+ CD45RO+ T lymphocytes during HIV-1 infection. Clin Exp Immunol 1994; 95:43641. First citation in article | PubMed
-
Behbahani H, Landay A, Patterson BK, et al. Normalization of immune activation in lymphoid tissue following highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2000; 25:1506. First citation in article | PubMed | CrossRef
-
Pluda JM, Yarchoan R, Smith PD, et al. Subcutaneous recombinant granulocyte-macrophage colony-stimulating factor used as a single agent and in an alternating regimen with azidothymidine in leukopenic patients with severe human immunodeficiency virus infection. Blood 1990; 76:46372. First citation in article | PubMed
-
Scadden DT, Pickus O, Hammer SM, et al. Lack of in vivo effect of granulocyte-macrophage colony-stimulating factor on human immunodeficiency virus type 1. AIDS Res Hum Retroviruses 1996; 12:11519. First citation in article | PubMed
-
Corica F, Allegra A, Corsonello A, et al. Relationship between plasma leptin levels and the tumor necrosis factor- system in obese subjects. Int J Obes Relat Metab Disord 1999; 23:35560. First citation in article | PubMed | CrossRef
-
Pernerstorfer-Schoen H, Jilma B, Perschler A, et al. Sex differences in HAART-associated dyslipidaemia. AIDS 2001; 15:72534. First citation in article | PubMed | CrossRef
-
Olsen NJ, Kovacs WJ. Gonadal steroids and immunity. Endocr Rev 1996; 17:36984. First citation in article | PubMed | CrossRef
-
Mynarcik DC, McNurlan MA, Steigbigel RT, Fuhrer J, Gelato MC. Association of severe insulin resistance with both loss of limb fat and elevated serum tumor necrosis factor receptor levels in HIV lipodystrophy. J Acquir Immune Defic Syndr 2000; 25:31221. First citation in article | PubMed | CrossRef
-
Ledru E, Christeff N, Patey O, de Truchis P, Melchior JC, Gougeon ML. Alteration of tumor necrosis factor T-cell homeostasis following potent antiretroviral therapy: contribution to the development of human immunodeficiency virusassociated lipodystrophy syndrome. Blood 2000; 95:31918. First citation in article | PubMed
-
Christeff N, Melchior JC, de Truchis P, Perronne C, Gougeon ML. Increased serum interferon alpha in HIV-1 associated lipodystrophy syndrome. Eur J Clin Invest 2002; 32:4350. First citation in article | PubMed | CrossRef
-
Maher B, Alfirevic A, Vilar FJ, Wilkins EG, Park BK, Pirmohamed M. TNF- promoter region gene polymorphisms in HIV-positive patients with lipodystrophy. AIDS 2002; 16:20138. First citation in article | PubMed | CrossRef
-
Bastard JP, Caron M, Vidal H, et al. Association between altered expression of adipogenic factor SREBP1 in lipoatrophic adipose tissue from HIV-1infected patients and abnormal adipocyte differentiation and insulin resistance. Lancet 2002; 359:102631. First citation in article | PubMed | CrossRef
-
Ziccardi P, Nappo F, Giugliano G, et al. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation 2002; 105:8049. First citation in article | PubMed | CrossRef
-
Hohler T, Kruger A, Gerken G, Schneider PM, Meyer zum Buschenefelde KH, Rittner C. A tumor necrosis factoralpha (TNF-) promoter polymorphism is associated with chronic hepatitis B infection. Clin Exp Immunol 1998; 111:57982. First citation in article | PubMed | CrossRef
-
Hohler T, Kruger A, Gerken G, Schneider PM, Meyer zum Buschenfelde KH, Rittner C. Tumor necrosis factor alpha promoter polymorphism at position -238 is associated with chronic active hepatitis C infection. J Med Virol 1998; 54:1737. First citation in article | PubMed | CrossRef
-
Balzarini J, Lee CK, Herdewijn P, De Clercq E. Mechanism of the potentiating effect of ribavirin on the activity of 2,3-dideoxyinosine against human immunodeficiency virus. J Biol Chem 1991; 266:2150914. First citation in article | PubMed
-
Kimball P, Elswick RK, Shiffman M. Ethnicity and cytokine production gauge response of patients with hepatitis C to interferon- therapy. J Med Virol 2001; 65:5106. First citation in article | PubMed | CrossRef
-
Fletcher CV, Acosta EP, Henry K, et al. Concentration-controlled zidovudine therapy. Clin Pharmacol Ther 1998; 64:3318. First citation in article | PubMed
-
Rea IM, McNerlan SE, Alexander HD. CD69, CD25, and HLA-DR activation antigen expression on CD3+ lymphocytes and relationship to serum TNF-, IFN-, and sIL-2R levels in aging. Exp Gerontol 1999; 34:7993. First citation in article | PubMed | CrossRef
-
Fletcher CV, Kawle SP, Kakuda TN, et al. Zidovudine triphosphate and lamivudine triphosphate concentrationresponse relationships in HIV-infected persons. AIDS 2000; 14:213744. First citation in article | PubMed | CrossRef
-
Carr A, Samaras K, Burton S, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS 1998; 12:F518. First citation in article | PubMed | CrossRef
-
Jones S, Janneh O, Maher B, Khoo S, Back D, Pirmohamed M. Altered TNF- and IL-6 levels and antiadipogenic effects of anti-retorivirals on cultured adipocytes: possible mechanisms for their role in lipodystrophy in HIV-infected patients. Antivir Ther 2002; 7:L2. First citation in article
-
Hoggard PG, Manion V, Barry MG, Back DJ. Effect of protease inhibitors on nucleoside analogue phosphorylation in vitro. Br J Clin Pharmacol 1998; 45:1647. First citation in article | PubMed | CrossRef
-
Lewis W, Haas CP, Raidel SM, et al. Combined antiretroviral therapy causes cardiomyopathy and elevates plasma lactate in transgenic AIDS mice. Lab Invest 2001; 81:152736. First citation in article | PubMed
-
Kleiner DE, Gaffey MJ, Sallie R, et al. Histopathologic changes associated with fialuridine hepatotoxicity. Mod Pathol 1997; 10:1929. First citation in article | PubMed
-
McKenzie R, Fried MW, Sallie R, et al. Hepatic failure and lactic acidosis due to fialuridine (FIAU), an investigational nucleoside analogue for chronic hepatitis B. N Engl J Med 1995; 333:1099105. First citation in article | PubMed | CrossRef
-
Cherry CL, Wesselingh SL. Nucleoside analogues and HIV: the combined cost to mitochondria. J Antimicrob Chemother 2003; 51:10913. First citation in article | PubMed | CrossRef
-
Cherry CL, McArthur JC, Hoy JF, Wesselingh SL. Nucleoside analogues and neuropathy in the era of HAART. J Clin Virol 2003; 26:195207. First citation in article | PubMed | CrossRef
-
Cote HC, Brumme ZL, Craib KJ, et al. Changes in mitochondrial DNA as a marker of nucleoside toxicity in HIV-infected patients. N Engl J Med 2002; 346:81120. First citation in article | PubMed | CrossRef
-
Pardo CA, McArthur JC, Griffin JW. HIV neuropathy: insights in the pathology of HIV peripheral nerve disease. J Peripher Nerv Syst 2001; 6:217. First citation in article | PubMed | CrossRef
-
Kotler DP. HIV infection and lipodystrophy. Prog Cardiovasc Dis 2003; 45:26984. First citation in article | PubMed | CrossRef
-
Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol 2000; 68:43746. First citation in article | PubMed
-
McGovern B, Bica I. Risk of HAART therapy in hepatitis C. Hepatology 2002; 35:730. First citation in article | PubMed | CrossRef
-
Hoggard PG, Veal GJ, Wild MJ, Barry MG, Back DJ. Drug interactions with zidovudine phosphorylation in vitro. Antimicrob Agents Chemother 1995; 39:13768. First citation in article | PubMed
-
Hoggard PG, Kewn S, Barry MG, Khoo SH, Back DJ. Effects of drugs on 2,3-dideoxy-2,3-didehydrothymidine phosphorylation in vitro. Antimicrob Agents Chemother 1997; 41:12316. First citation in article | PubMed
-
Brau N. Update on chronic hepatitis C in HIV/HCV-coinfected patients: viral interactions and therapy. AIDS 2003; 17:227990. First citation in article | PubMed
-
Becher F, Pruvost A, Goujard C, et al. Improved method for the simultaneous determination of d4T, 3TC and ddl intracellular phosphorylated anabolites in human peripheral-blood mononuclear cells using high-performance liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 2002; 16:55565. First citation in article | PubMed | CrossRef
-
Dabis F, Ekpini ER. HIV-1/AIDS and maternal and child health in Africa. Lancet 2002; 359:2097104. First citation in article | PubMed | CrossRef
|
|
| |
| |
|
|
|