| |
Fat Distribution in HIV-Infected Women in the United States: DEXA Substudy in the Women's Interagency HIV Study
|
| |
| |
JAIDS Jan 2005
".....In summary, this cross-sectional study in a well-characterized cohort of mostly overweight or obese women showed that leg fat is lower in HIV-infected women on HAART, regardless of whether they are on a PI-containing or PI-sparing regimen. Trunk fat was also significantly lower in women on a non-PI-containing HAART regimen, but not in those on PI-based HAART. In contrast to studies in cohorts of HIV-infected men, increasing age was not associated with lower levels of peripheral fat. The clinical implications of peripheral fat loss in the context of overweight or obesity require further study....
......Total, trunk, and leg fat were all significantly lower in the HIV-positive women, but there was no difference between groups in total lean body mass (LBM)...
.... These results portray a different pattern from other papers on fat distribution in HIV-infected women, which have focused primarily on clinical reports of central fat accumulation, as well as objective measurements of higher levels of fat in the trunk....
.... This cross-sectional study of fat distribution among women in the WIHS cohort provides evidence of significantly lower levels of leg fat in HIV-infected women on HAART, when compared with both HIV-negative women and HIV-positive women who are not currently using ART...
.... results suggest that there is no effect of HIV infection per se on fat distribution in this population, consistent with results reported previously in men...
..... In contrast, in both groups of women on HAART, leg fat tended to be proportionally lower than trunk fat, suggestive of a disproportionate reduction in leg or peripheral fat in both HAART-treated groups. Our results could also be said to suggest that there is greater relative retention of trunk or central fat in the HAART/PI group (90% of controls), but not in the HAART/no PI group (77% of controls).....
..... Among HIV-positive women, the finding that nadir CD4+ lymphocyte count was positively and significantly associated with leg fat is consistent with clinical observations from the HIV Outpatient Study....
.....Conversely, in contrast to surveys in groups that were predominantly male, in which increasing age has consistently been a potent predictor of lipoatrophy, age was not a significant factor in total or regional fat distribution in the present study. In fact, age >30 tended to be associated with more rather than less fat.....
...... It must be acknowledged that DEXA cannot distinguish between visceral and subcutaneous fat in the abdominal region, and it is possible that fat distribution within the trunk could have differed in ways that were not detectable by DEXA. In contrast, in the arms and legs, most of the fat is subcutaneous, so leg fat measured by DEXA primarily represents total subcutaneous fat content in that region......
JAIDS Journal of Acquired Immune Deficiency Syndromes: Volume 38(1) 1 January 2005
Mulligan, Kathleen PhD*†; Anastos, Kathryn MD‡; Justman, Jessica MD§; Freeman, Ruth MD‖; Wichienkuer, Paula BS*¶; Robison, Esther PhD‡; Hessol, Nancy A. MSPH*
From the *University of California, San Francisco, CA; †Division of Endocrinology, San Francisco General Hospital, San Francisco, CA; ‡Montefiore Medical Center, Bronx, NY; §Bronx-Lebanon Hospital Center, Bronx, NY; ‖Albert Einstein College of Medicine, Bronx, NY; and ¶Oregon Health and Science University, Portland, OR.
Summary:
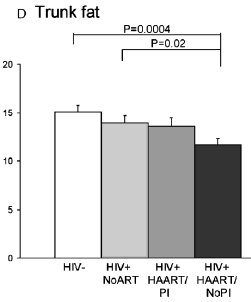
Surveys in HIV-infected men on antiretroviral therapy (ART) consistently demonstrate decreased levels of peripheral fat, with variable effects on central fat. This substudy of the Women's Interagency HIV Study was undertaken to examine fat distribution in a well-characterized cohort of HIV-positive and HIV-negative women in the United States. Whole-body dual-energy x-ray absorptiometry scanning with standardized regional analysis was performed in 271 nonpregnant women. Results were compared in the following groups: HIV negative (n = 88); and HIV positive on no ART (n = 70), highly active ART with a protease inhibitor (HAART/PI) (n = 48), or non-PI-containing HAART (n = 53). The groups were well matched with respect to race, with the majority of women coming from racial/ethnic minorities. The majority of both HIV-positive and HIV-negative women were overweight (body mass index [BMI] >=25 kg/m2), and many were obese (BMI >30 kg/m2). Leg fat in both groups on HAART was significantly lower than in HIV-negative women (P = 0.01 and <0.0001 vs. HIV-negative for HAART/PI and HAART/no PI, respectively), whereas trunk fat was lower only in HAART/no PI (P = 0.0004 vs. HIV-negative). Thus, consistent with reports in men, lower levels of peripheral (leg) fat are seen in HIV-infected women on HAART, despite the high prevalence of obesity in this population.
INTRODUCTION
Alterations in fat distribution, including both central fat accumulation and peripheral fat loss, have been widely documented in HIV-infected populations with access to highly active antiretroviral therapy (HAART). To date, most studies have been performed in groups that were primarily or exclusively men whose pre-HIV body habitus tended to be normal or slender.1-3 In contrast, many HIV-infected women in the United States are overweight or obese, and the effects of HIV infection and HAART in the context of preexisting obesity are not known. The present study was undertaken to explore the associations of HIV infection and antiretroviral therapy (ART) with fat distribution, measured by dual-energy x-ray absorptiometry (DEXA), in a well-characterized cohort of HIV-positive and HIV-negative women with a high prevalence of obesity; and to examine other disease and lifestyle factors that impact on fat distribution in this setting.
RESULTS
Subject Characteristics
A total of 88 HIV-negative and 183 HIV-positive women were enrolled. The groups were well matched in terms of racial/ethnic distribution, with the majority of women in both groups identifying themselves as members of racial or ethnic minorities. The HIV-positive women were slightly, but significantly, older than HIV-negative women. There were no differences between groups in self-reported exercise level, education level, number of male or female sex partners, or the proportion of women who reported trading sex for drugs or money (data not shown).
Body Mass and Composition: HIV Positive vs. HIV Negative
The majority of women in both groups had a BMI in the overweight or obese categories. Overall, both weight and BMI in the HIV-positive women were significantly lower than in the HIV-negative women. This difference between groups in weight was explained entirely by differences in fat content. Total, trunk, and leg fat were all significantly lower in the HIV-positive women, but there was no difference between groups in total lean body mass (LBM).
Evaluation of HIV-Positive Women by Treatment Group
Among the HIV-positive women, women on HAART/no PI were slightly younger (39 ± 1 vs. 42 ± 1 years in the no ART group; P = 0.008). There were no differences in current CD4+ count (411 ± 40, 504 ± 36, and 448 ± 39 cells/μL in no ART, HAART/PI, and HAART/no PI, respectively), whereas nadir CD4+ count was significantly higher in no ART (289 ± 27 vs. 206 ± 24 and 210 ± 157 cells/μL in no ART, HAART/PI, and HAART/no PI, respectively; P = 0.02 and 0.04 for no ART vs. HAART/PI and HAART/no PI, respectively). The median HIV-1 RNA level tended to be higher and the range of values greater in no ART, but differences between groups were not statistically significant. The groups did not differ with respect to racial distribution, cigarette smoking, menopausal status, exercise level, or other lifestyle factors. The predominant NRTIs in HAART/PI and HAART/no PI, respectively, were lamivudine (75 and 71%), zidovudine (35 and 54%), stavudine (52 and 37%), and abacavir (15 and 46%), with more limited use of didanosine and tenofovir. The distribution of PI use in HAART/PI was 42% nelfinavir, 25% lopinavir/ritonavir, 15% indinavir, 10% each ritonavir and saquinavir, and 6% amprenavir. Nineteen percent of the women in HAART/PI were also on an NNRTI. In the HAART/no PI group, 48% were on efavirenz, 25% on nevirapine, and the remainder on at least 3 NRTIs including abacavir. Among current HAART users, the average number of 6-month intervals or their equivalent during which HAART use was reported was 6.1. Thirty-one percent of women in the no ART group had prior antiretroviral exposure, but they had been off ART for an average of 17 months at the time of study.
Body Composition
Total fat was significantly lower in the HAART/no PI group than in either the HIV-negative or HIV-positive no ART groups (Fig. 1A). The difference between the HAART/PI and HIV-negative groups in total fat approached but did not achieve a level of statistical significance (P = 0.06). Total LBM was remarkably consistent across groups (Fig. 1B). Leg fat in both groups on HAART was significantly lower than in HIV-negative women (Fig. 1C). Leg fat in HAART/no PI was also significantly lower than in the untreated HIV-positive women. Trunk fat was lower only in HAART/no PI (Fig. 1D). In a post hoc analysis, leg fat in HAART/no PI was significantly lower than in HAART/PI (P = 0.04), and trunk fat tended to be lower in HAART/no PI (P = 0.07). (note from jules Levin: there appears to be a trend in reduced trunk fat for HIV+ vs HIV-.):
|
|
| |
| |
 |
|
| |
| |
Multivariate Analysis
In multivariate analysis of factors associated with total and regional fat in HIV-positive women, exercise level (>6 hours per week) was the only factor associated with lower levels of total as well as both trunk and leg fat. Among women who reported exercising >6 hours per week, the most frequently reported activities were (in decreasing order) walking, dancing, cycling, and weight lifting. Race had no significant effect on trunk fat, but African American women had significantly more leg fat than those in other racial/ethnic groups. Smoking and nadir CD4+ count were associated with lower total and leg fat. Current CD4+ count had no significant association with total or regional fat (data not shown). Duration of stavudine was significantly associated with the amount of leg fat but not trunk fat. No such association was seen with zidovudine or lamivudine. Neither current nor maximum levels of HIV-1 RNA were associated with differences in total or regional fat and were not included in the final analysis. Although more HIV-positive women had a history of pregnancy, were postmenopausal, and were coinfected with hepatitis C virus, none of these factors was significant in multivariate analyses of influences on total or regional fat. Inclusion of menopausal status in the GLM models did not affect the borderline significance of age as a factor in higher levels of total and trunk fat.
AUTHOR DISCUSSION
This cross-sectional study of fat distribution among women in the WIHS cohort provides evidence of significantly lower levels of leg fat in HIV-infected women on HAART, when compared with both HIV-negative women and HIV-positive women who are not currently using ART. These differences are seen against the backdrop of a high prevalence of overweight and obesity, which are common in US women, particularly members of racial and ethnic minorities. Because the control group in the WIHS cohort was selected to be well matched for race/ethnicity, risk behavior, and other demographic factors, it is reasonable to suggest that the pre-HIV body habitus of the HIV-positive women was similar to that of the controls and that the observed differences in BMI and total and regional fat are a result of HIV infection, ART, and other factors associated with the disease.
In the HIV-positive group on no ART, mean values for both leg and trunk fat were approximately 90% of those in HIV-negative controls, showing no differences in proportional fat distribution. These results suggest that there is no effect of HIV infection per se on fat distribution in this population, consistent with results reported previously in men. In contrast, in both groups of women on HAART, leg fat tended to be proportionally lower than trunk fat, suggestive of a disproportionate reduction in leg or peripheral fat in both HAART-treated groups. Our results could also be said to suggest that there is greater relative retention of trunk or central fat in the HAART/PI group (90% of controls), but not in the HAART/no PI group (77% of controls).
These results portray a different pattern from other papers on fat distribution in HIV-infected women, which have focused primarily on clinical reports of central fat accumulation, as well as objective measurements of higher levels of fat in the trunk. In fact, it could be argued that the results of the present study (lower amounts of leg, ie, peripheral, fat and conservation or relatively less of a reduction in central fat) are more comparable to those described in the initial report from Carr et al in a cross-sectional study in a group of subjects who were predominantly male, white, and lean.
Among HIV-positive women, the finding that nadir CD4+ lymphocyte count was positively and significantly associated with leg fat is consistent with clinical observations from the HIV Outpatient Study.3 Conversely, in contrast to surveys in groups that were predominantly male, in which increasing age has consistently been a potent predictor of lipoatrophy, age was not a significant factor in total or regional fat distribution in the present study. In fact, age >30 tended to be associated with more rather than less fat. The association of exercise with differences in regional fat warrants further investigation. It is unlikely that exercise can fully explain the between-group differences in leg fat, as there were no significant differences among HIV-positive groups in exercise level. It would be of interest to see if these same factors affect fat distribution in nonobese women.
It must be acknowledged that DEXA cannot distinguish between visceral and subcutaneous fat in the abdominal region, and it is possible that fat distribution within the trunk could have differed in ways that were not detectable by DEXA. In contrast, in the arms and legs, most of the fat is subcutaneous, so leg fat measured by DEXA primarily represents total subcutaneous fat content in that region.
In summary, this cross-sectional study in a well-characterized cohort of mostly overweight or obese women showed that leg fat is lower in HIV-infected women on HAART, regardless of whether they are on a PI-containing or PI-sparing regimen. Trunk fat was also significantly lower in women on a non-PI-containing HAART regimen, but not in those on PI-based HAART. In contrast to studies in cohorts of HIV-infected men, increasing age was not associated with lower levels of peripheral fat. The clinical implications of peripheral fat loss in the context of overweight or obesity require further study.
METHODS
Subjects
The Women's Interagency HIV Study (WIHS) is a multicenter prospective study initiated in 1994 and designed to comprehensively investigate the natural history of HIV-1 infection in women in the United States.4 For this substudy, 271 women at 2 WIHS sites (Bronx/Manhattan, n = 218, and San Francisco, n = 53) were recruited between April 2001 and March 2003. Women who were pregnant, had type I diabetes, used exogenous hormones or systemic steroids, or were receiving pharmacologic treatment of osteoporosis were excluded, as were women who weighed >122 kg (the upper limit for the DEXA scanners). The protocol was approved by the institutional review boards at each site, and written informed consent was obtained from all participants prior to enrollment.
Data Collection
Women were asked to fast for at least 8 hours before the study visit. Height and weight were measured after subjects had removed their shoes and heavy clothing. Body mass index (BMI) was calculated as weight (kg) divided by height (m2), and results were classified using World Health Organization criteria. Whole-body DEXA scanning was performed on Lunar Corp (Madison, WI) models Prodigy (Bronx) or DPX (San Francisco) instruments, following standardized calibration and positioning procedures. Post hoc manual regional analysis of the DEXA scans was performed following the same standardized procedure at each research site. Some subjects did not fully fit within the DEXA scan area, so small portions of their arms were not scanned. Accordingly, we report values for leg fat as an index of peripheral fat content in all participants.
Participants were interviewed about their current ART regimens and menopausal status. They were asked to estimate he number of hours per week that they engaged in exercise and indicate what types of exercise they performed routinely. Data on ART history, smoking habits, current and nadir CD4+ lymphocyte count, HIV-1 RNA, coinfection with hepatitis B or C, and social and medical history were obtained from the parent study. Duration of ART for most women was calculated as the number of 6-month intervals during which they reported use of each agent since enrolling in the WIHS.
Data Analysis and Statistical Methods
After examining differences between HIV-positive and HIV-negative women, we subdivided the HIV-positive group according to current ART use as follows: those who were using o ART at the time of study (no ART, n = 70); those on a PI-containing HAART regimen (HAART/PI, n = 48); and those on a HAART regimen that did not include a PI (HAART/no PI, n = 53). Twelve HIV-positive women were on ART regimens that did not meet the criteria for HAART7 and are not included the analyses of differences in HIV-positive women.
For continuous variables, data were plotted and checked for outliers and normality of distribution using the Shapiro-Wilk statistic. Unpaired t tests and f statistics were used to compare the distribution of study characteristics by HIV status. Unpaired t tests were used to compare results in HIV-negative women to each group of HIV-positive women; and, among HIV-positive women, to compare results in those on no ART with each treatment group. For multivariate analyses we used the generalized linear model (GLM) since there were unequal numbers of observations for every combination of the classification factors (covariates). The dependent variables for the GLM analyses were total, trunk, and leg fat, adjusted for height. The covariates included in the final models were those that remained statistically significant. Data are reported as the mean ± SE. All statistical analyses were performed using SAS software, version 8 (Cary, NC).
|
|
| |
| |
|
|
|