 |
 |
 |
| |
Study Finds EPO Improved SVR Only in High Dose RBV Group
|
| |
| |
Reported by Jules Levin
The results of this study reported at AASLD Nov 2005 did not find EPO improved SVR rates nor did it increase the average dose of RBV in patients who took EPO compared to patients who did not take EPO when standard weight-based dosing of RBV was used; although hemoglobin declines to <10 g/dl were reduced, and maximal HB declines were reduced, and the percent of patients with RBV dose reductions was reduced, and the mean dose reduction was less (27 mg/d vs 179 mg/d) in patients receiving standard weight-based dosing of RBV. Still, mean RBV dose (mg/d) was the same in the group receiving EPO & group not receiving EPO among patients receiving standard weight-based RBV dosing. ONLY in the patients receiving RBV high weight-based dosing (1000-1600 mg/d) was the use of EPO found to improve SVR rates. I'm not sure I am convinced by this study, something seems not right here but I can't put my finger on it.
"TREATMENT OF CHRONIC HCV GENOTYPE 1 WITH PEGINTERFERON ALFA-2B (PEGIFN), HIGH WEIGHT BASED DOSE RIBAVIRIN (RVN) AND
EPOETIN ALFA (EPO) ENHANCES SUSTAINED VIROLOGIC RESPONSE (SVR)"
Mitchell L. Shiffman, Virginia Commonwealth University Medical Center, Richmond, VA; Angie Price, Sarah Hubbard, Mary Wilson, Jennifer Salvatori, Virginia Commonwealth University Medical Center, Richmond, VA; Richard K. Sterling, Virginia Commonwealth University Medical Center, Richmond, VA; R
T. Stravitz, Virginia Commonwealth University Medical Center, Richmond, VA; Velimir A. Luketic, Virginia Commonwealth University Medical Center, Richmond, VA; Arun J. Sanyal, Virginia Commonwealth University Medical Center, Richmond, VA
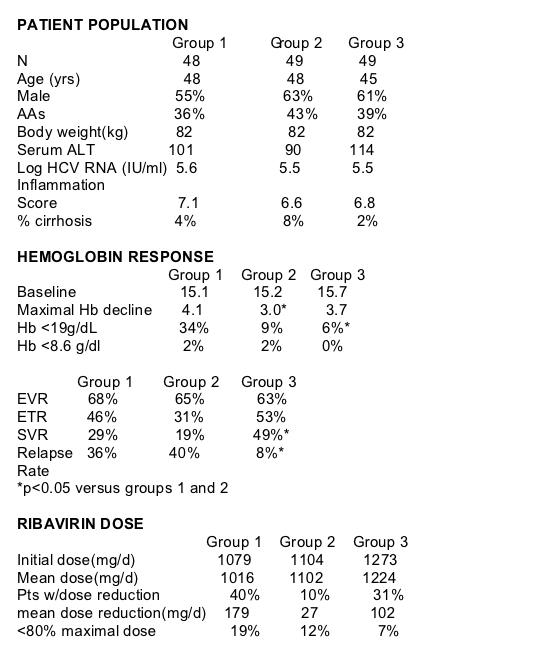
There were 3 patient study groups in this study:
150 patients with HCV genotype 1, naıve to prior treatment, were randomly assigned to one of three treatment groups (50/group):
--Group 1: PEGIFN alfa-2b (1.5 mcg/kg/wk) + weight based RVN (WBRBN) 13.3 mg/kg/day (800-1400 mg/day);
--Group 2: PEGIFN alfa-2b + WBRVN + EPO (40,000 U/wk) or
--Group 3: PEGIFN alfa-2b + a higher dose of WBRVN (HDWBRVN) 15.2 mg/kg/day (1000-1600 mg/day) + EPO.
Hypothesis for study: Successful treatment of chronic HCV with PEGIFN and RVN is hampered by adverse events (AEs). One of the most common AEs is anemia and this frequently requires that RVN either be dose reduced or prematurely discontinued. RVN dose reduction, particularly during the first 24 weeks of therapy, is associated with a significant reduction in SVR; particularly in patients with HCV genotype 1. Our hypothesis was that utilizing EPO at the onset of PEGIFN and RVN therapy would reduce the frequency of anemia, the need to dose reduce RVN, allow for use of higher RVN dosing and this in turn could enhance SVR.
In groups 2 and 3 EPO was initiated at the onset of therapy if hemoglobin was <15 gm/dl as soon as the Hb <15 g/dl. Starting RPO dose was 40,000 U/week and titrated between 20,000 –60,000 U/wk to maintain the hemoglobin (Hb) between 12-15 gm/dl. EPO dose was increased to 60,000/wk if Hb did not increase 1 gm/dl after 4 weeks. EPO dose was held if Hb >15 g/dl. In all groups, the RVN dose was reduced by 200 mg increments if the Hb declined below 10 gm/dl or for management of other side effects as needed, and discontinued in patients who developed severe hemoglobin <8.6 g/dl or severe AEs not responding to dose reduction. All patients underwent liver biopsy prior to treatment. HCV RNA was assessed by Taqman PCR.
Patients were excluded for:
Any other co-existant liver disorder besides hcv
Decompensated cirrhosis
Hemoglobin less than 11 gm/dl
WBC <3,000
HIV+platelet count <80,000
Recipient of an organ or tissue transplant
RESULTS:
This study found that SVR was improved by use of EPO only in the high dose RBV group and not in the standard weight-based dose RBV group when both groups receiving EPO were compared to standard RBV weight-based dosing group who did NOT receive EPO, despite a reduction in anemia in the standard RBV dose group who received EPO. This appeared to be due to a great reduction in the relapse rate in the high dose RBV group only.
AUTHOR SUMMARY
The use of EPO at the initiation of HCV therapy significantly reduced:
--Frequency of anemia
--The need for RBV dose reduction
--The mean dose by which RBV was reduced
HOWEVER, this did not significantly effect:
--overall mean RBV dose
--early viraologic response
--sustained virologic response
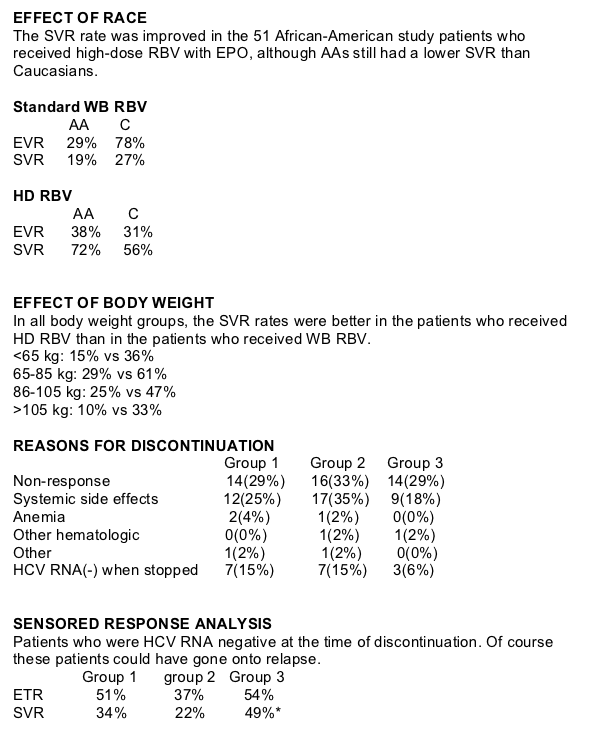
The use of higher dose weight-based RBV (1,000-1,600 mg/d) with EPO at the start of HCV therapy significantly increased SVR in patients with HCV genotype 1:
-across all body weights
-in both whites and blacks
The increase in SVR was achieved:
-despite the need to dose reduce ribavirin in 31 percent of patients
-significant reduction in relapse occurred.
|
|
| |
| |
|
 |
 |
|
|