| |
Care of patients with chronic hepatitis B and HIV co-infection: recommendations from an HIV-HBV International Panel
|
| |
| |
AIDS: Volume 19(3) 18 February 2005 p 221-240
Soriano, Vincenta; Puoti, Massimob; Bonacini, Maurizioc; Brook, Garyd; Cargnel, Antoniettae; Rockstroh, Juergenf; Thio, Chloeg; Benhamou, Yvesh
From the aDepartment of Infectious Diseases, Hospital Carlos III, Madrid, Spain
bClinica di Malattie Infettive e Tropicali, Spedali Civili, Brescia, Italy
cGastroenterology/Hepatology, Department of Transplantation, California Pacific Medical Center, San Francisco, CA, USA
dDepartment of Genitourinary Medicine, Central Middlesex Hospital, London, UK
eDivisione di Malattie Infettive, Ospedale Luigi Sacco, Milan, Italy
fDepartment of Medicine, University of Bonn, Bonn, Germany
gDivision of Infectious Diseases, Viral Hepatitis Center, Johns Hopkins University, Baltimore, MA, USA
hHepatology and Gastroenterology Unit, Hôpital Pitié Salpétriêre, Paris, France.
Sponsorship: Funding was kindly provided by Gilead, Roche, Schering-Plough, GlaxoWellcome and Bristol-Myers-Squibb.
Introduction
Liver disease caused by chronic hepatitis B virus (HBV) infection is currently an important cause of morbidity and mortality among HIV-infected patients in the western world, where classical opportunistic complications of severe immunodeficiency have declined dramatically as a result of the widespread use of potent antiretroviral therapies [1,2]. Over the past few years, several consensus reports have addressed the issue of viral hepatitis and HIV co-infection. However, as a result of the larger impact of hepatitis C virus (HCV), they have focused mainly on HIV and HCV co-infection [3], whereas only a few reports have devoted particular attention to hepatitis B [4,5]. There are several reasons to highlight HBV in HIV-positive individuals (Table 1).
The large amount of new information on HBV and HIV, as well as important changes made in the most recent guidelines for using anti-HBV drugs [6,7] and antiretroviral compounds [8], prompted us to organize a consensus conference, which was held in San Francisco, USA, in February 2004, to update knowledge and formulate recommendations on how to provide the best care to HBV/HIV-co-infected patients. Following international recommendations for the development of clinical guidelines [9], the meeting was planned as a workshop in which experts in the field of HIV and viral hepatitis discussed a total of 14 questions, which were selected in advance by the panelists as the most relevant and conflicting topics in the management of chronic hepatitis B in the setting of HIV infection (Table 2). A draft was written and circulated among panel members during the following months. Finally, a consensus was reached and is presented here. The statements are graded according to the Infectious Disease Society of America scoring system [9], with minor changes. Briefly, the quality of evidence for any statement was graded as 1 (based on properly randomized, controlled trials), 2 (based on other kinds of publications), and 3 (based on expert opinion). The strength of the recommendation was categorized as A (good), B (modest) or C (poor).
Burden of HIV and hepatitis B virus co-infection
Background
Whereas 40 million individuals are estimated to be infected with HIV worldwide, nearly 400 million people are chronic HBV carriers [10]. HBV and HIV are endemic in the same world regions and share their routes of transmission, although HBV is more infectious than HIV [1,4]. Accordingly, co-infection with both viruses is frequently seen, with most co-infected individuals living in sub-Saharan Africa and in the far east. In western countries, the prevalence of chronic HBV infection is overall 10-fold higher among HIV-positive individuals than in the general population. Patients infected through male homosexual contact tend to show the highest rates, approaching 10%, whereas they are slightly lower among intravenous drug users (IDU) and individuals infected through heterosexual contact [1,4,11-15].
Serological evidence of previous exposure to HBV is found in more than 80% of HIV-positive patients, with considerable variation according to the geographical region and risk group [1,2,4]. Atypical serum HBV markers are common among HIV-infected individuals. Isolated anti-hepatitis B core antigen is frequently recognized [16,17], particularly among patients with more severe immune deficiency. These patients are at risk of HBV reactivation, and in one study [18], transient hepatitis B surface antigen (HBsAg) appeared in 24% and serum HBV-DNA in 60% of these individuals on prolonged follow-up. Moreover, approximately one third of them had histologically confirmed liver damage.
Eight major genotypes of HBV have been described (named A to H), with more than 8% nucleotide divergence each based on the complete genomic sequence [19-21]. HBV genotypes show a characteristic geographical distribution (Table 3) and may influence the natural history of liver disease and the response to antiviral agents [20,21]. As in HIV-negative individuals, HBV genotypes A and D are predominant among HBV/HIV-co-infected patients in north America and western Europe [20-25]. Interestingly, genotype A affects mainly homosexual men whereas genotype D is predominant seen among IDU in southern Europe [22,23]. Patients with genotype A tend to be hepatitis B e antigen positive (HBeAg), whereas those with genotype D are more frequently HBeAg negative [22]. Treatment response to interferon seems to be better among genotype A patients.
Panel summary
Chronic hepatitis B affects nearly 10% of HIV-infected patients, and therefore approximately 4 million people worldwide are HBV/HIV-co-infected. Atypical HBV serological markers may be recognized, particularly among those with more severe immunosuppression. HBV genotypes may differ among different risk groups and distinct geographical regions, which may influence the natural history of liver disease and the treatment outcome. SCORE: A1.
Liver disease caused by hepatitis B virus in HIV-infected patients
Background
HBV is a small hepatotropic DNA, non-cytopathic virus that causes liver damage mainly through immune-mediated mechanisms [26,27]. Cytotoxic CD8 T cells recognize HBV antigens in the context of HLA class I exposed on the surface of infected hepatocytes and destroy them. The killing of these cells results in elevated aminotransferase levels. Sustained chronic hepatic inflammation may lead to liver cirrhosis. In contrast with HCV, which replicates in the cytosol of infected hepatocytes, HBV establishes a persistent infection with a stable reservoir of its genetic material as a covalently closed circular DNA (cccDNA) within the nucleus of the infected hepatocytes. In this way, HBV resembles HIV (whose genetic material integrates into the chromosomes of the target cells) mmore than HCV. However, in contrast to HIV, which needs reverse transcription for its integration into the host genome, in HBV infection reverse transcription is needed for replication of the HBV-DNA material through a long RNA intermediate.
Although most HIV-negative individuals with chronic hepatitis B do not develop hepatic complications, 25-30% do go on to develop serious HBV-related disease during their lifetime, including liver failure and hepatocellular carcinoma [28]. The risk of HBV-associated end-stage liver disease seems to be increased in the setting of HIV co-infection [1,2]. The presence of HIV infection increases the risk of chronicity after exposure to HBV [29]. Moreover, it reduces the rate of spontaneous HBsAg and HBeAg seroconversion [29]. The prevalence of HBeAg-negative chronic hepatitis B as well as the HBV inactive carrier state thus tends to be lower in HBV/HIV-co-infected individuals. Finally, although higher serum HBV-DNA levels are seen in co-infected patients [29], a recent report has shown that HBV-DNA titres do not increase shortly after HIV seroconversion in previous HBV carriers [30].
Despite higher serum HBV-DNA levels, hepatic necro-inflammation tends to be milder in HBV/HIV-co-infected individuals [31], which is in agreement with the postulated immune-mediated pathogenicity of HBV. However, the enhanced replication levels of HBV in HIV-co-infected patients may result paradoxically in the progression of more severe liver fibrosis [32]. Consistent with this observation, several clinical studies have shown that the risk of end-stage liver disease is significantly increased in HIV-infected patients with chronic hepatitis B [33-35], mimicking what is happening in HCV/HIV co-infection [3,36]. The risk of more rapid liver disease progression should be particularly considered in HIV/HBV-co-infected patients with detectable HBV-DNA, with or without HBeAg. In contrast, in HIV-positive patients with chronic HBV infection, lacking evidence of HBV replication (inactive carrier state), this risk could be negligible [37].
The possibility that diminished immune responses in HIV-infected individuals might lead to higher rates of HBV escape has been a matter of controversy over the past years when considering patients lacking HBsAg [38,39]. These 'silent' or 'occult' HBV infections, as defined by the presence of detectable HBV-DNA levels in the liver (and occasionally in the serum) in HBsAg-negative patients, might have clinical implications. Hypothetically, it might enhance the risk of hepatotoxicity using antiretroviral drugs, reduce the response to anti-HCV treatment, and even lead to liver damage in an occult fashion. Given the immune deficiency status caused by HIV, occult hepatitis B could be expected to be more frequent in HIV carriers. However, recent studies have not found evidence of such 'occult' HBV infections, testing HBV-DNA in the serum with sensitive techniques in HBsAg-negative, HIV-infected patients with markers of previous HBV exposure [40,41]. Therefore, the prevalence and potential impact of 'occult' hepatitis B infections are still unclear in the setting of HIV infection.
Panel summary
Elevated serum HBV-DNA levels are frequently seen in HBV/HIV-co-infected patients, whereas liver enzyme elevations are frequently milder than in patients with chronic HBV monoinfection. Liver histological damage may progress more rapidly in HBV/HIV-co-infected patients, leading to cirrhosis in a shortened timeframe. These patients show an increased risk of HBV-related liver complications and death. SCORE: B1.
Is hepatitis B virus a co-factor for HIV disease progression?
Background
A persistent state of immune activation is seen in patients with chronic HBV replication [26], as occurs in HCV carriers [27]. This circumstance as well as a putative direct effect of some HBV factors on HIV transcription [42] might favour an enhanced HIV replication, which could lead to faster CD4 T-cell declines in HIV/HBV-co-infected individuals.
The majority of clinical studies conducted so far have examined the influence of HBV on HIV disease progression, considering only HBsAg as a marker of chronic HBV infection. They have provided conflicting results [1,37,43], although most of them have not proved an independent association between HBV co-infection and a worse course of HIV disease. As chronic 'replicative' HBV infection requires the presence of substantial amounts of HBV-DNA in the serum besides HBsAg, further studies examining specifically the outcome of HBsAg-positive patients with high HBV-DNA titres should be conducted to determine to what extent HBV may exert a direct deleterious effect on the natural history of HIV infection.
Panel summary
Chronic hepatitis B with evidence of active HBV replication might act as a co-factor for HIV disease progression. HBV might accelerates HIV disease progression indirectly, enhancing immune activation. However, no definitive proof for a role of HBV on HIV disease progression has been reported so far. SCORE: C3.
Hepatitis B virus treatment: when, goals and monitoring
As HBV infection is currently not readily eradicable [21,27], the objective of treatment is to delay and ideally stop liver disease progression, thereby preventing the development of liver cirrhosis and hepatocellular carcinoma [5,7]. Five different parameters are generally used to assess HBV-related liver damage and to monitor treatment response: serum HBV-DNA, HBeAg, HBsAg, alanine aminotransferase (ALT) and liver histology. Taking these into consideration, the goals of HBV treatment may be categorized into several steps, from less to more ambitious [5]. First, treatment should pursue the suppression of HBV replication, as reflected by the achievement of significant reductions or clearance of serum HBV DNA. Second, therapy may shift HBV infection from HBeAg-positive to anti-hepatitis B e. Third, ideally any anti-HBV therapy should pursue the disappearance of serum HBsAg and the development of anti-HBsAg. However, this goal is very difficult to achieve in clinical practice because the pool of the major transcriptional template of HBV in the liver, the cccDNA, escapes the direct antiviral effect of most anti-HBV drugs. Fourth, therapy should provide a reduction of liver inflammation and then a reduction or normalization of ALT and a halt in the progression of liver fibrosis, with an improvement in liver histology. In most instances, the prolonged suppression of HBV replication leads to histological improvement, induces a significant decrease or normalization of aminotransferase levels and prevents disease progression to cirrhosis and end-stage liver disease. Regression of liver cirrhosis with anti-HBV therapy has been reported sporadically [44].
Other benefits of HBV therapy include the reduction in the risk of transmission and of evolution to chronicity after acute HBV infection [45]. In the case of HIV-infected patients, HBV treatment may also decrease the risk of highly active antiretroviral therapy (HAART)-related hepatotoxic events [1].
Chronic HBV infection in adults generally consists of an early replicative phase with active liver disease (HBeAg positive) and a late low or non-replicative phase with HBeAg seroconversion and remission or inactivation of liver disease [46]. After HBeAg seroconversion, some patients may continue to show active HBV replication because of the selection of HBeAg-defective viral variants. Two major groups of mutations result in HBeAg-negative chronic hepatitis. One occurs in the pre-core region and another in the basic core promoter region. Table 4 summarizes the current classification of chronic hepatitis B status. After an average of 30 years, 25% of patients with chronic active hepatitis B will progress to liver cirrhosis [47]. Without therapy, approximately 15% of these patients with cirrhosis will die within 5 years, as survival is rather poor among decompensated cirrhotic individuals [46].
In HBV-monoinfected patients, the indication for therapy until recent years often relied on histological findings. As liver disease evolves faster and more severely in HIV/HBV-co-infected patients and as the therapeutic armamentarium has expanded rapidly, the use of pharmacological interventions has been revisited and may now be considered without knowing the result of liver histology, relying only on the information provided by other HBV disease markers, such as ALT, HBeAg and serum HBV DNA. The indication for HBV therapy is currently based mainly on these markers, and liver biopsy is generally only considered for particular situations.
The biochemical goal (ALT normalization) is initially achieved in most cases with any anti-HBV agents. Elevated transaminases are a marker of liver inflammation. Higher ALT levels correlate with the risk of cirrhosis [47-50]. In HBeAg-negative chronic hepatitis B patients, both high ALT and serum HBV-DNA levels are associated with active disease [50,51]. On the other hand, high pre-treatment ALT values correlate with better treatment responses to either interferon or lamivudine. For example, in HBV-monoinfected patients the rate of HBeAg seroconversion was 64, 34 and 5% in patients with ALT values greater than fivefold, two to fivefold and less than twofold, respectively, using lamivudine [52].
The value of ALT as a surrogate treatment marker, however, is limited because of the following: fluctuations are common, lower levels may still be associated with liver disease progression, and poor correlation exists between ALT levels and inflammatory scores, particularly in HBeAg-negative patients. Furthermore, ALT elevations in HIV/HBV-co-infected individuals may be a result of many factors (i.e. antiretroviral agents, HCV, alcohol, immune reconstitution, etc.) and the correlation between ALT levels and liver disease may be even lower [32].
The serological goal (HBeAg or HBsAg seroconversion) is only achieved by a minority of patients treated with anti-HBV agents [53-57]. Table 5 summarizes the results obtained in HBV-monoinfected patients. HBeAg loss induced by IFN-α treatment is durable in more than 80% of these patients after more than 5 years of follow-up [7,58]. The data on the durability of the response to lamivudine are not so encouraging, and relapses often occur when lamivudine is discontinued soon after HBeAg loss, particularly among Asian patients [59,60]. Cumulative evidence suggest that lamivudine should be prolonged for at least 6 months after HBeAg seroconversion to minimize the risk of relapse upon drug discontinuation [7,61]. More encouraging results in terms of the durability of seroconversion have been reported with adefovir, which has also been shown to reduce the cccDNA pool [56]. The ultimate endpoint of HBsAg loss is observed in only 5-10% of patients within one year of the start of IFN-α treatment, but occurs less frequently with nucleos(t)ide analogues.
HBeAg is generally regarded as a marker of HBV replication, and in the past patients found to be HBeAg negative were considered to have non-replicative HBV infection. However, specific mutations were identified in the late 1980s that accounted for the recognition of HBV viraemia in patients without HBeAg. The most common is at the precore region, which produces a stop codon and prevents HBeAg production. Currently, the precore mutant HBV strain is the major cause of chronic hepatitis B in the Mediterranean area. HBeAg-negative chronic hepatitis B is generally not transmitted and is therefore only rarely acquired as a de novo infection. More frequently, the precore mutant emerges as the predominant species during the course of typical HBV infection, and is selected during the immune clearance phase (HBeAg seroconversion). HBeAg-negative chronic hepatitis can occur either close to HBeAg seroconversion or many years later. Sustained remission is rarely achieved in HBeAg-negative chronic hepatitis B, and the long-term prognosis is poor in most of these patients [7,51].
The virological goal (serum HBV-DNA reduction) is initially obtained using most anti-HBV agents. In HBV-monoinfected individuals, there is a linear correlation between serum HBV-DNA titers, ALT levels and histological inflammatory activity, but less so with liver fibrosis [62]. Classically, patients with serum HBV-DNA levels above 105 copies/ml and elevated ALT levels were considered to have active HBV disease, whereas individuals with lower HBV loads had inactive infection. However, HBeAg-negative patients typically show lower HBV-DNA levels, as well as one third of HBeAg-positive individuals [62-65]. A reduction in serum HBV DNA in response to therapy correlates with an improvement in the histological activity index [62], with HBeAg seroconversion, and with a reduced risk of selecting drug resistance.
The HBV-DNA threshold associated with progressive HBV-related liver disease is unknown. Recent guidelines in HIV-negative individuals have recommended that treatment must be considered for HBeAg-positive patients with detectable serum HBV-DNA levels above 105 copies/ml (100,000), whereas lower HBV-DNA thresholds should be considered for HBeAg-negative patients and those with decompensated cirrhosis (thresholds of 104 (10,000) and 103 (1000) copies/ml, respectively) [7].
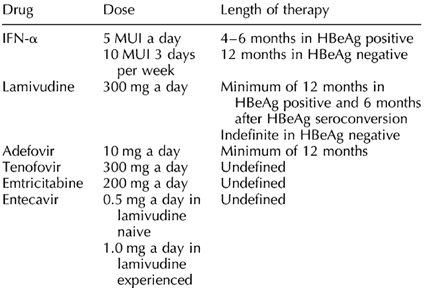
At this time, no strict recommendations can be made about which patients with HBV/HIV co-infection should be treated. The decision should be based on several considerations. The main consideration is the need for HAART. If HAART is already in use or is needed in drug-naive patients presenting with low CD4 cell counts, the inclusion of anti-HIV drugs with further anti-HBV activity (such as lamivudine, emtricitabine or tenofovir) seems to be the best choice. In contrast, if HAART is not needed, the use of anti-HBV agents without anti-HIV activity (i.e. IFN-α or adefovir at doses of 10 mg per day) might be preferred (Fig. 4). No agents with both anti-HBV and anti-HIV activity (i.e. lamivudine, emtricitabine or tenofovir) should be used as monotherapy, because of the high risk of selecting HIV-resistant variants. More data are needed to ensure that adefovir at low doses do not select resistance mutations in HIV, which might compromise the future activity of tenofovir.
In HIV-infected patients not requiring HAART, IFN-α may be the best option for HBeAg-positive chronic active hepatitis, given that it provides the best response rates. In contrast, adefovir 10 mg a day might be the first option for HBeAg-negative patients not requiring antiretroviral treatment. The duration of adefovir treatment is still undefined and may be lifelong. Therefore, definitive histological evidence of advanced liver damage might be required before recommending treatment in these patients. Optionally, in HBeAg-negative patients with mild liver disease (no septa), a watchful strategy might be advisable. Combination therapy with pegylated IFN-α and adefovir is currently being investigated in HIV-negative patients, although preliminary results have not shown any advantage over pegylated IFN-α alone.
In HIV-infected patients with CD4 cell counts of less than 350 cells/μl and therefore requiring HAART or in patients already on antiretroviral therapy, the use of agents with double anti-HIV and anti-HBV activity is advisable. When possible, combination treatment with a nucleoside analogue (lamivudine or emtricitabine) plus tenofovir should be administered. These combinations may provide greater antiviral efficacy and delay the selection of drug-resistant strains. Finally, an earlier prescription of HAART with those agents may be discussed on an individual basis in patients whose criteria to initiate antiretroviral therapy [8] have still not been fulfilled.
Histological information is certainly relevant for therapeutic decisions. However, the role of liver biopsy, particularly in the setting of HBV/HIV co-infection, has been reassessed since the availability of new, more potent and well-tolerated anti-HBV agents [5,7,28]. HBV therapy may now be considered in the absence of histological data, on the basis of serological markers alone. However, initial and further treatment options, length of therapy, etc. may be influenced when the severity of the HBV-related liver lesion is known. Therefore, histological information should be pursued any time and a liver biopsy should currently be recommended in HBV/HIV-co-infected individuals, although should not be mandatory before initiating HBV therapy.
Among the advantages of liver biopsy is that it is a widely available procedure that assesses the severity of necrosis, inflammation and fibrosis. In addition, it helps to rule out other causes of liver damage (opportunistic agents, drug toxicity, alcohol, steatosis, etc.). Finally, in HIV/HBV-co-infected patients, liver cirrhosis may exist despite normal ALT levels [32], supporting the convenience of a liver biopsy to assess patients with repeatedly normal transaminase levels (Fig. 3). However, sampling variations, the different expertise of pathologists, and the risk of complications are among the major limitations of liver biopsy [66-68]. No less relevant, a patient's reluctance to accept a liver biopsy often represents an important obstacle and should not be dismissed. Fortunately, over the past few years, non-invasive methods including biochemical tests [69,70] and new imaging techniques [71,72] have been shown to provide an adequate estimation of the extent of liver fibrosis. For example, the so-called 'fibro-test' uses five serum markers and has been shown to predict and differentiate accurately mild and severe liver fibrosis in patients with chronic hepatitis B and C, with and without HIV co-infection [73-75]. Finally, as new therapies provide better response rates, limiting the prescription of treatment only to those with significant liver histological damage may not be advisable, and drugs may begin to be more widely used at earlier stages [76]. However, while expecting the results of ongoing clinical trials with the new anti-HBV drugs, whose long-term safety and efficacy are so far unknown, a watchful waiting strategy could be considered in patients with HBeAg-negative chronic hepatitis B with no evidence of fibrous septa. Following this course, a knowledge of the liver disease stage could be useful for making appropriate therapeutic decisions in these patients.
Panel summary
Anti-HBV therapy should be considered for all HIV/HBV-co-infected patients with any evidence of liver disease (i.e. elevated transaminase levels, elevated HBV-DNA titers, necro-inflammatory lesions and fibrosis in the liver biopsy) irrespective of the prevailing CD4 cell count. In HIV/HBV-co-infected patients not requiring HAART, HBV therapy should be preferentially based on interferon or adefovir. In contrast, in patients presenting with CD4 cell counts of less than 350 cells/μl or those already on antiretroviral therapy, the use of agents with double anti-HIV and anti-HBV activity should be preferred. When possible, combination treatment with a nucleoside analogue (lamivudine or emtricitabine) plus tenofovir should be administered. An earlier prescription of HAART with those agents may be discussed on an individual basis in patients whose criteria to initiate antiretroviral therapy have still not been fulfilled. In HBeAg-negative patients with early liver fibrosis stage and without a need for beginning antiretroviral therapy, a watchful waiting strategy could be advisable instead of immediate anti-HBV treatment. SCORE: B2.
Interferon alpha
IFN-α was the first drug approved for the treatment of chronic hepatitis B. Its efficacy is greater in HBeAg-positive than in HBeAg-negative patients. The doses recommended are 5-6 MU a day or 9-10 MU three times a week for 4-6 months in HBeAg-positive and 5-6 MU three times a week for 12 months in HBeAg-negative patients [1,6,7,28]. Patients with high ALT levels and low HBV-DNA titers show the best responses.
A meta-analysis has shown lower chances of response to IFN-α in HIV/HBV-co-infected patients [77], with HBeAg seroconversion rates below 10% and even lower in the absence of controlled HIV replication, and with significant CD4 cell declines while on IFN-α therapy.
Since the availability of the new pegylated forms of IFN-α, much interest been shown about its efficacy in the treatment of chronic hepatitis B. In HIV-negative patients, recent data have proved that pegylated interferon is more effective than standard IFN-α in HBeAg-positive chronic hepatitis B [78]. With respect to HBeAg-negative individuals, pegylated IFN-α is more effective than lamivudine, and the combination of pegylated IFN-α plus lamivudine does not seem to improve the benefits of pegylated IFN-α monotherapy [79].
Given the side-effects of IFN-α, it is generally prescribed only for relatively short periods of time (6-12 months), whereas nucleos(t)ide analogues may be administered indefinitely. However, the rate of HBeAg seroconversion is generally higher using IFN-α than using nucleos(t)ide analogues, particularly in HBeAg-positive patients. Moreover, IFN-α efficacy in HIV/HBV-co-infected patients seems to be greater in individuals with higher CD4 cell counts. For these reasons, IFN-α should be the preferred option for treating HBeAg-positive chronic hepatitis B in HIV-co-infected patients when no requirement for antiretroviral therapy exists. However, in patients with liver cirrhosis, necroinflammatory flares accompanying HBeAg clearance may lead to liver decompensation, and therefore IFN-α should be used cautiously in this subset of patients. In patients with decompensated liver disease, IFN-α is contraindicated.
Panel summary
The efficacy of IFN-α therapy is greater in HBeAg-positive than in HBeAg-negative chronic hepatitis B. However, the response rate is much lower in HIV-co-infected patients. The poor tolerance of IFN-α usually limits its use, and CD4 cell drops may be of particular concern in HIV-positive individuals. Results using pegylated IFN-α alone or in combination with nucleos(t)ide analogues are awaited. In the meantime, IFN-α may be the best choice for HIV/HBV-co-infected patients not requiring antiretroviral therapy. However, IFN-α should be used cautiously in HBeAg-positive patients with advanced liver disease and is contraindicated in patients with decompensated cirrhosis. SCORE: B3.
Nucleoside analogues: lamivudine and emtricitabine
The primary goal of HBV therapy is the durable suppression of serum HBV-DNA to the lowest level possible [80]. In patients who are HBeAg-positive an additional goal of treatment is the loss of HBeAg with seroconversion to anti-HBeAg. The latter is preferable because the achievement of complete HBeAg seroconversion indicates that antiviral treatment may be stopped. Given that the response to IFN-α is much lower in HIV-co-infected patients, and particularly among those with low CD4 cell counts, the new availability of oral compounds with both anti-HIV and anti-HBV activities has offered new hope for these patients, who will often need indefinite treatment.
Two different families of polymerase inhibitors are available for the treatment of chronic hepatitis B, nucleoside and nucleotide analogues. Lamivudine was the first nucleoside analogue approved for the treatment of chronic hepatitis B. The effective anti-HBV dose is 100 mg a day, whereas 300 mg a day is the dose approved for HIV therapy. In both HIV-negative and HBV/HIV-co-infected patients, lamivudine therapy is associated with significant HBV suppression, an improvement in liver function tests and liver histological findings, and the reversal of liver decompensation. The overall rate of HBV-DNA reduction below the limit of detection of hybridization assays (105 copies/ml) in response to lamivudine monotherapy is between 40 and 87% in HIV/HBV-co-infected patients [81-83]. The e seroconversion rates were 22-29% in these 3 studies.
The selection of HBV variants with lamivudine resistance (YMDD mutants) may be associated with acute exacerbation (severe flare of serum ALT and HBV DNA), that can lead to fulminant liver failure [84-86]. In addition, a reversion of histological improvement and progression of liver disease occurs in most patients after selecting lamivudine-resistant strains [87-91]. When lamivudine resistance is suspected, the addition of a second anti-HBV agent rather than its replacement is preferred by many experts, although the Gilead 461 trial did not show any benefit of keeping lamivudine with adefovir. However, as approximately 10% of patients seem to show a lack of susceptibility to adefovir, for reasons that are still unclear, a period of 3 months under both drugs seems to be worthwhile before discontinuing lamivudine, and with clear evidence of HBV suppression by adefovir.
Emtricitabine has recently been approved for the treatment of HIV, and is being evaluated as an anti-HBV agent [92]. The effective dose for HBV seems to be 200 mg once a day [93], which is the same dose approved for HIV therapy. Like lamivudine, emtricitabine is a cytosine analogue with both anti-HIV and anti-HBV activity. Its similarity to lamivudine makes it appropriate in terms of tolerance and dosing as long as no lamivudine resistance has been selected by either HIV or HBV, given that these compounds share cross-resistance [94].
At different doses (25-300 mg a day), emtricitabine has been shown in HBV-monoinfected patients to produce an intense and rapid HBV-DNA reduction (mean of -3.4 log in 56 days) [93]. The low level of HBV DNA is still maintained after 48 weeks of treatment in more than half the patients. The drug is well tolerated with no dose-limiting adverse events [92,93]. Preliminary clinical results suggest that resistance to emtricitabine may occur less frequently than with lamivudine [95].
Panel summary
Lamivudine and emtricitabine are pyrimidine analogues with excellent safety profiles, and both anti-HIV and anti-HBV activities. They should be considered interchangeable and not additive. Given their low genetic barrier for resistance, they should not be used as monotherapy in HIV/HBV-co-infected patients. They are particularly recommended in patients on antiretroviral therapy and in those taking other concomitant antiretroviral drugs, particularly in HBeAg-negative chronic hepatitis B patients, who will often need prolonged periods of therapy. SCORE: A1.
Nucleotide analogues: adefovir and tenofovir
In contrast to nucleoside analogues, nucleotide analogues are phosphorylated pro-drugs, and are active against lamivudine-resistant HBV strains [96-98]. Two are currently available as anti-HBV agents: adefovir and tenofovir.
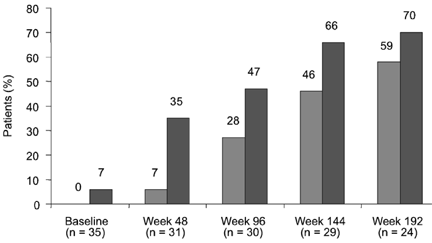
Adefovir was the first approved nucleotide analogue for the treatment of chronic hepatitis B. The dose recommended is 10 mg a day, which does not show activity against HIV. In one study conducted in 35 HIV/HBV-co-infected patients with lamivudine-resistant HBV, the addition of adefovir to lamivudine provided a significant reduction in serum HBV-DNA levels, which was maintained for 192 weeks, along with the normalization of transaminases in most cases (Fig. 6) [99]. In up to 144 weeks of therapy, adefovir resistance mutations had not been selected in HBV or in HIV. These results are similar to those obtained in HBV-monoinfected patients [61,100], although a mutation at codon rt236 has been shown to be selected in nearly 2% of HBV-monoinfected patients on adefovir after 2 years of follow-up [101,102]. In-vitro studies have confirmed that this change is associated with a significant reduction in HBV susceptibility to adefovir [101]. Another mutation, A181V, has also recently been associated with adefovir resistance [102]. Importantly, adefovir-resistant HBV remains sensitive to lamivudine, emtricitabine and entecavir [102]. Recent reports, however, have suggested that some HIV/HBV-co-infected patients may show a lack of susceptibility to adefovir in the absence of resistance mutations. Interestingly, all these patients responded to subsequent tenofovir treatment [103].
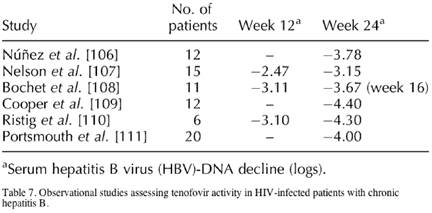
Lamivudine along with adefovir and IFN-α are the only drugs so far approved for the treatment of hepatitis B. As already highlighted, the rapid development of resistance represents a major drawback for lamivudine monotherapy. This is particularly worrisome in HIV-positive patients, in whom lamivudine is a frequently prescribed antiretroviral drug, and in whom HBV-resistant strains seem to be selected more rapidly [83]. For these reasons, there is much interest in the use of other oral anti-HBV compounds, to be used either alone or in combination with lamivudine. In-vitro data have shown additive to synergistic antiviral effects of adefovir when combined with other anti-HBV nucleoside analogues [104]. However, given that tenofovir, but not adefovir, has been approved and is already widely used as an antiretroviral agent, its potent anti-HBV activity [105] has attracted much attention. During the past year, several in-vitro [106] and in-vivo [107-113] studies have demonstrated that tenofovir is active against lamivudine-resistant HBV strains (Table 7). In an extended follow-up, additional results in support of those findings have been reported, with 70% of patients showing undetectable serum HBV-DNA levels after 2 years on tenofovir and 15% of patients showing HBeAg seroconversion [114].
In addition to being used as a rescue intervention in individuals with lamivudine-resistant HBV strains, tenofovir is being investigated alone as part of a combination therapy with lamivudine and emtricitabine in drug-naive individuals. These combinations may increase anti-HBV activity and may lead to a reduced risk of selecting YMDD mutant viruses. In the Gilead 903 trial, which compared tenofovir versus stavudine in HIV-positive drug-naive individuals who initiated HAART with a backbone of efavirenz and lamivudine, a retrospective study in 11 HBsAg-positive patients has recently been conducted [115]. After 144 weeks of therapy, the mean reduction in serum HBV-DNA levels among the six subjects receiving tenofovir plus lamivudine was of 4.5 log copies/ml. while it was of 1.9 log copies/ml in the five patients in the lamivudine monotherapy arm. All five patients on lamivudine monotherapy for HBV developed lamivudine-resistant strains, whereas resistance only developed in one out of six subjects receiving tenofovir plus lamivudine. Despite the retrospective nature of the study and the relatively small number of patients examined, the data argue in favour of the use of combination therapy over monotherapy for the treatment of hepatitis B, at least in HIV-co-infected patients. A recent report in six French HBV/HIV-co-infected patients has also confirmed the great efficacy of the dual combination of tenofovir and lamivudine in drug-naive patients [116]. Clearly, these data suggest that combination therapy for HBV could be beneficial to enhance antiviral activity as well as for delaying the selection of HBV-resistant strains. Whereas this benefit may be seen combining some of the new potent drugs, no encouraging data in favour of combination therapy have been obtained so far when using IFN-α, lamivudine or adefovir together, which are the only currently approved anti-HBV agents. Table 8 summarizes the therapeutic armamentarium against HBV, including approved antiretroviral agents with anti-HBV activity and drugs such as entecavir, which is soon expected to be available.
Fig 6. Adefovir for 3TC resistant HBV. HBV DNA <1000 copies/ml.
|
|
| |
| |
 |
|
| |
| |
The use of combination therapy for HBV infection is currently under investigation. Whereas some combinations have proved to provide greater antiviral activity, others have not. For example, in one study conducted in HIV-negative patients [117], adefovir alone was compared with adefovir and lamivudine given together, and the combination was not found to be more potent than adefovir alone in the first year of treatment. At this time, combination therapy should be explored further clinically, especially for patients who are unable to achieve complete HBV-DNA suppression during monotherapy.
|
|
| |
| |
 |
|
| |
| |
PANEL SUMMARY
|
|
| |
| |
 |
|
| |
| |
Adefovir at a dose of 10 mg a day is active against HBV but not against HIV. In contrast, tenofovir at a dose of 300 mg a day is active against both viruses. These drugs show a more robust genetic barrier to resistance than lamivudine and emtricitabine. Moreover, adefovir resistance mutations in HBV are selected at different positions than for lamivudine or emtricitabine. When possible, combination therapy with one nucleoside and one nucleotide analogue should be preferred to monotherapy with any of these drugs in HIV/HBV-co-infected patients. SCORE: B2.
New anti-hepatitis B virus drugs
Background
The new compounds being tested for the treatment of HBV infection may be grouped into two categories. The first includes drugs active against both HBV and HIV. Another group includes medications with activity against HBV alone. Compounds from the latter group might be preferentially indicated in individuals who have not yet met the criteria for beginning HIV therapy. More than 15 molecules with potential activity against HBV are under investigation. Several of them are already in the final steps of clinical development, such as entecavir, clevudine and telbivudine. Overall, they show much greater antiviral potency, and may produce a decline of cccDNA in hepatocytes, which so far has only been seen for adefovir but not for lamivudine.
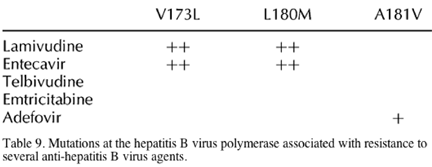
Entecavir is probably the next drug to be approved for the treatment of HBV infection. It is a deoxyguanosine analogue specific for HBV and lacks any anti-HIV activity. It is one of the most potent anti-HBV agents examined so far [118,119] and shows a relatively good safety profile [119]. Phase 3 trials are ongoing worldwide, including a few in HIV/HBV-co-infected patients. Virological responses are seen in patients with lamivudine-resistant HBV, although they tend to be slightly lower than in lamivudine-naive patients. Resistance to entecavir seems to result from the accumulation of multiple changes in the HBV polymerase, including those causing lamivudine resistance (Table 9) [120]. For this reason, entecavir doses of 0.5 mg a day are recommended in drug-naive patients, but doses of 1.0 mg a day are preferred for patients with previous exposure to lamivudine [121,122]. Moreover, a dose of 1.0 mg a day will probably be recommended for HBV/HIV-co-infected individuals. Given the good safety profile of entecavir, including the absence of mitochondrial toxicity, and its lack of anti-HIV activity, it is likely that this drug will be the first oral product to manage HBV infection independently in HIV/HBV-co-infected patients.
Clevudine is a pyrimidine nucleoside analogue. Different doses were tested in 31 HBV-monoinfected patients [122]. A 3 log viral load reduction was observed at week 4 of therapy, with normalization in ALT values in approximately 70% of patients [123].
Telbivudine is an L-nucleoside analogue of thymidine. At different doses (400 and 600 mg a day) it produces a more profound HBV-DNA reduction than lamivudine at 52 weeks in HBV-monoinfected patients [122,124]. However, the combination of lamivudine and telbivudine does not produce higher virological suppression than telbivudine alone. Moreover, those drugs share cross-resistance (Table 9). The rate of anti-HBeAg seroconversion appeared to be higher with telbivudine than with lamivudine (33 versus 28%), and lower when telbivudine and lamivudine are combined (17%). Finally, the proportion of patients who normalize transaminase levels is higher with telbivudine than with lamivudine (86 versus 63%) [124].
|
|
| |
| |
 |
|
| |
| |
Panel summary
New nucleoside analogues show strong anti-HBV activity and do not inhibit HIV. They may be used as monotherapy in HBV/HIV-co-infected patients not taking antiretroviral drugs. Entecavir, clevudine and telbivudine are the most promising agents, with a good safety profile. The activity of clevudine and telbivudine is halted in the presence of YMDD mutants, whereas the activity of entecavir is only slightly reduced in the face of lamivudine resistance. None of these compounds show cross-resistance with adefovir or tenofovir. Particularly in combination, they may further improve efficacy and avoid viral breakthroughs. SCORE: C2.
Hepatotoxicity of antiretroviral drugs in hepatitis B virus carriers
Background
Significant liver enzyme elevations occur on average in 5-10% of HIV-positive patients who start triple antiretroviral therapy [125]. The rate is higher in patients with underlying chronic hepatitis B [126-132]. Moreover, some drugs (i.e. nevirapine, efavirenz or ritonavir at full doses) cause hepatotoxicity more frequently than the rest [125,133]. Liver function tests should thus be closely monitored in patients who initiate antiretroviral treatment, particularly when some of the drugs mentioned above are administered to individuals with chronic hepatitis B.
Cumulative toxicity may explain the steady liver enzyme elevations when using some compounds. If not apparent shortly after beginning therapy, it may be manifested much later, often after 4-6 months on therapy. This has been seen with drugs such as nevirapine [134-136].
Liver enzyme elevations as a result of antiretroviral treatment may occur by other mechanisms than the direct injury of the drug(s) prescribed. Immune reconstitution phenomena and hypersensitivity reactions may account for some additional cases [137]. In patients with low CD4 cell counts or high HIV-RNA titers, successful anti-HIV therapy may enhance the immune responses to such a degree that hepatic cells harbouring HBV antigens may be recognized and destroyed massively. As long as the patient remains asymptomatic and transaminase levels do not rise above 10 times the limit of normal values (grade 4 toxicity), treatment could be continued with close monitoring of laboratory values, as the return of liver enzymes to baseline values or even complete HBV clearance may occur [137,138]. On the other hand, allergic phenomena that may develop shortly after exposure to nevirapine, abacavir or amprenavir may be accompanied by liver enzyme elevations in the context of a more generalized reaction. The presence of underlying chronic hepatitis B does not seem to play a role in the occurrence of this phenomenon [136].
Liver toxicity may also occur as a consequence of mitochondrial damage in patients receiving nucleoside analogues, particularly zidovudine, stavudine or didanosine. Histological features of hepatic steatosis are more common in women, obese individuals, and when two of these drugs are taken together or for long periods [139,140].
Panel summary
Liver enzyme elevations after beginning antiretroviral therapy are more frequent in patients with underlying chronic hepatitis B. Therefore, drugs with more hepatotoxic profiles (i.e. nevirapine, efavirenz, full-dose ritonavir) should be used cautiously in co-infected patients. Treatment should be discontinued in patients with symptoms or grade 4 increases in aminotransferase levels. In certain cases, immune reconstitution phenomena may lead to liver enzyme elevations after starting HAART. Close monitoring of these patients during the first weeks may allow them to be kept on therapy, because they tend to experience a progressive resolution of liver abnormalities without discontinuing treatment. Mitochondrial toxicity of some nucleoside analogues (mainly zidovudine, stavudine or didanosine) may result in steatohepatitis. SCORE: A2.
Prevention of further liver damage in hepatitis B virus carriers
Liver function can further deteriorate in HIV/HBV co-infected patients as a result of other hepatotropic viruses (hepatitis A, C, D, E), the administration of drugs other than antiretroviral agents, or substance abuse, including alcohol.
There is an increased rate of severe/fulminant hepatitis after acute hepatitis A virus (HAV) infection in HBV carriers [141]. HAV vaccination should thus be provided to all individuals with chronic hepatitis B without serum anti-HAV IgG. The vaccine response is lower in patients with low CD4 cell counts. HAV immunization should be performed as early as possible, and in patients who have low CD4 cell counts, re-vaccination should be considered after the CD4 cell levels have risen with HAART [142].
Fulminant hepatitis, more aggressive liver disease and hepatocellular carcinoma are more frequent in the presence of chronic hepatitis B and concomitant infection with HCV or hepatitis D virus (HDV) [143-146]. The effect is generally additive rather than multiplicative. Although the transmission of HCV and HDV is usually parenteral, sexual transmission may also occur [147]. Therefore, as there are no vaccines to protect from infection with HCV or HDV, preventative measures are important, such as avoiding risky injecting practices and unprotected sex.
There is an additive effect of alcohol and HBV in terms of the evolution to fulminant hepatitis, aggressive chronic liver disease and hepatocellular carcinoma [148]. This relationship is linear and proportional to the extent of alcohol intake and duration. There is no 'safe' threshold above which alcohol begins to be deleterious, although more than 60 g a day is often considered risky. The encouragement of alcohol withdrawal with psychological support and professional detoxification programmes driven by specialists, and including the use of drugs when appropriate (i.e. acamprosate, naltrexone) is of benefit and should be advised [149].
Chronic HBV carriers are more prone to develop hepatotoxicity after exposure to some drugs. In HBV/HIV-co-infected patients, particular attention should be paid to anti-tuberculous agents [150]. Isoniazid, rifampin and pyrazinamide, particularly when taken together, often result in liver enzyme elevations, and force treatment withdrawal. The use of alternative agents is often required to treat these patients.
Panel summary
All non-immunized HBV carriers should be vaccinated for HAV. HAV serostatus should be checked in pre-vaccinated individuals and if low or absent titers are found, re-vaccination should be offered, particularly to patients with rising CD4 cell counts in response to HAART. Proper hygiene counseling should be offered to avoid exposure to HAV and hepatitis E virus. With respect to HCV and HDV, for which there are no prophylactic vaccines, counseling should be focused on avoiding risky injection practices and unprotected sex. When necessary, pharmacological and psychological support should be instituted to reduce alcohol consumption. Hepatotoxic drugs, such as anti-tuberculous agents, should be given with caution. SCORE: B2.
Hepatitis B virus vaccination: indications and schedules
Background
The screening of serum HBV markers should be requested after the first HIV diagnosis. Individuals lacking HBV markers should be vaccinated. In the case of negative HAV markers, combination vaccination is advisable. In patients with more advanced immunodeficiency, the response to the HBV vaccine is much poorer and extra doses are often needed to obtain protective anti-hepatitis B surface antibody (HBsAb) titers (>100 IU/ml) [151,152]. Moreover, periodic monitoring of these antibodies is warranted because they may decline progressively over time, and may put the patient at risk of acute infection in the case of exposure [153].
HIV-positive patients develop weaker humoral responses to HBV vaccine, especially if the CD4 cell count is below 500 cells/μl, and lose protective antibodies faster. After three vaccine doses, the response rate is 87% in HIV-positive patients with CD4 cell counts greater than 500 cells/μl, but is only 33% in patients with CD4 cell counts between 200 and 500 cells/μl [154]. Ongoing viral replication and concomitant immune system activation in HIV-infected patients decreases the ability of B lymphocytes to respond to HBV vaccination [151].
HBV vaccination should start with the conventional dose (20 μg at months 0, 1, and 6-12) for patients with CD4 cell counts greater than 500 cells/μl. In individuals with CD4 cell counts between 200 and 500 cells/μl, an intensive schedule is recommended (20 μg at months 0, 1, 2 and 12). Patients who do not respond to the first cycle should receive booster doses or a new vaccination cycle with 40 μg at months 0, 1, 2 and 6-12, until they show detectable serum anti-HBsAb, ideally greater than 100 IU/l. Patients with CD4 cell counts of less than 200 cells/μl who are not on antiretroviral therapy should receive HAART first and thereafter HBV immunization, preferentially after the CD4 cell count has increased above 200 cells/μl. After successful immunization, anti-HBsAb levels should be checked yearly and booster doses should be given to those with anti-HBsAb levels of less than 100 IU/l [155].
Panel summaryHBV vaccination should be provided to all HIV-positive individuals with no serum HBV markers. In the case of negative HAV markers, combination vaccination is advisable. The response to HBV vaccine is lower in HIV-positive individuals, particularly among those with lower CD4 cell counts. Anti-HBsAb titers should be checked 12 weeks after ending the vaccination cycle and booster doses or extra cycles are advisable when no appropriate humoral responses have been obtained. In patients with CD4 cell counts of less than 500 cells/μl, intensive schedules are initially warranted. SCORE: A2.
Diagnosis and management of hepatitis B virus reactivations
Background
The occurrence of liver enzyme flare-ups in HIV-positive individuals with chronic hepatitis B is not a rare phenomenon. They may occur in two different settings. First, in HBsAg-positive patients with complete serum HBV suppression while taking antiretroviral therapy including drugs with anti-HBV activity. The interpretation of HBV reactivations in these individuals may be difficult because many reasons may account for it (see Table 10). For example, the discontinuation of lamivudine (emtricitabine) or tenofovir for any reason [156-158] or the development of lamivudine (emtricitabine) resistance by HBV (which occurs in 30-50% of HIV-infected patients after 12 months of therapy) [83] may be accompanied by abrupt transaminase flares [159], which may be wrongly interpreted as hepatotoxicity of current antiretroviral drugs. A rapid improvement in the immune function as a result of potent antiretroviral therapy may also be involved in flares of clinical hepatitis in these patients [160].
On the other hand, hepatitis B reactivation has been reported in HIV-infected individuals who had fully recovered from HBV infection, developing anti-HBsAb [161]. It has also been seen in patients with isolated anti-hepatitis B core positivity [162]. HBV reactivations are accompanied by an increase in serum transaminase levels, and the reappearance of serum HBsAg and an increase in HBV DNA. Although HBV reinfection may be the cause of these episodes, reactivation of the previous infecting HBV variant is the most frequent cause. Accordingly, reactivation in these patients is often seen in those who have received immunosuppressive agents for treating neoplasias, such as lymphomas or Kaposi's sarcoma [163,164]. More rarely, HBV reactivations are seen in patients presenting with very low CD4 cell counts. These reactivations indirectly support the notion that HBV establishes a longstanding infection that is never truly eradicated. Therefore, in HIV-infected patients with serum markers of past HBV infection, pre-emptive therapy should be considered for minimizing the risk of HBV reactivation in the case of cancer chemotherapy. The use of nucleos(t)ide analogues rather than IFN-α seems to be most appropriate in this setting.
PANEL SUMMARY
Table 10. causes of liver enzyme elevations in hepatitis b surface antigen positive HIV+ patients.
|
|
| |
| |
 |
|
| |
| |
HIV-infected patients with serum markers of previous hepatitis B (anti-HBsAb or anti-hepatitis B core antibody) or HBsAg-positive carriers with complete suppression of HBV replication under antiviral agents may experience abrupt flare-ups in transaminase levels, which may occasionally be fatal. In most instances, HBV reactivations rather than reinfections are the cause. The withdrawal of anti-HBV agents, the development of resistance, or the use of immunosuppressive agents often account for most of these episodes. SCORE: B3.
Treatment in patients with multiple viral hepatitis (hepatitis C or hepatitis D)
Background
Multiple chronic hepatitis virus infections are not rare in HIV-infected patients, because most of these agents share their transmission routes. This is particularly true among IDU, in whom more than 75% of chronic hepatitis B patients show anti-HCV antibodies. Moreover, HDV often accompanies HCV in the Mediterranean basin and other prevalent regions for the virus.
Although chronic hepatitis caused by multiple viruses tends to be more aggressive than hepatitis caused by single agents, the interaction between these agents is complex [165]. In patients without immunosuppression, HDV is almost always driving liver damage in the case of multiple hepatitis virus infections. In uncontrolled HIV infection, multiple virus escape often exists, with no evidence of inhibitory competition or interference between hepatotropic viruses. High serum HDV-RNA titers and delta antigen are often seen in the bloodstream of HIV-positive patients, along with markers of HBV or HCV replication [166,167]. As a result of this complex interaction, conflicting data have been reported for liver histology in HIV-positive patients with hepatitis B accompanied by HDV or HCV infections [166-170], although evolution towards liver cirrhosis tends to be faster and the outcome is generally much worse in these multiply co-infected patients than in individuals with monoinfections [146,170,171]. Therefore, the treatment of patients with multiple hepatitis viruses is warranted. However, responses tend to be poor and the most convenient therapeutic schemes are unclear at this time. The treatment of HDV with high doses of IFN-α plus or minus lamivudine and for long periods is rarely followed by sustained HDV-RNA clearance [172-174]. The use of anti-HBV nucleoside analogues alone generally does not confer any benefit to patients with hepatitis delta [175]. Finally, co-infection with HCV rarely responds to combination therapy with IFN-α plus ribavirin when delta virus is present.
Panel summary
Chronic hepatitis B is not rarely accompanied by serological evidence of hepatitis C and D virus infections, particularly among IDU. In patients with uncontrolled HIV infection, the escape (replication) of multiple viruses rather than competition between them is often seen. More severe liver damage in patients with multiple hepatitis leads to a greater urgency to treat them; however, convenient drugs, doses and schedules have not yet been defined, and overall response rates tend to be much worse than for single hepatitis virus infections. SCORE: C3.
Management of end-stage hepatitis B virus-related liver disease: screening for hepatocellular carcinoma and liver transplantation
Background
HIV-infected patients with end-stage liver disease caused by HBV develop classic complications of decompensated liver cirrhosis, including ascites, jaundice, gastrointestinal bleeding, spontaneous peritonitis and encephalopathy. Moreover, these patients are at risk of developing hepatocellular carcinoma. Occasionally, this tumour may develop without cirrhosis, although advanced fibrosis is found in most instances. In the setting of HIV infection, hepatocellular carcinoma may appear at a younger age and may be more aggressive [176]. Screening for this neoplasia with ultrasonography and alphafetoprotein should be performed in all HBV/HIV cirrhotic patients every 6 months.
In patients with end-stage HBV-related liver disease, the only treatment available is orthotopic liver transplantation (OLT). Initial attempts before the introduction of HAART regimens provided very poor results [177,178]. Those reports showed that only a small percentage of transplanted HIV-positive recipients maintained good organ function, whereas most experienced an accelerated course to AIDS [177,178]. Since the introduction of HAART, HIV-infected liver transplant recipients have improved their short and mid-term survival [177,180]. Now, the outcome of transplantation is no longer compromised as long as HIV infection is controlled with HAART in the post-transplant period.
The criteria currently used for liver transplantation in HIV-positive patients includes the following: no previous history of opportunistic infections, CD4 cell counts greater than 100-200 cells/μl and undetectable plasma HIV-RNA levels on HAART (or available drugs for successful treatment in the post-OLT period). In the literature there have been at least 10 HIV-positive patients with end-stage liver disease caused by chronic hepatitis B who underwent OLT and all remained alive, in some cases up to 3 years [179,180]. A poorer prognosis has been reported for HCV [179,180], although this difference has only been reported in comparing patients without HIV co-infection, and is mainly a result of the almost universal HCV recurrence in the allograft followed by rapid progression to liver cirrhosis in nearly 20% of cases within 5 years [181]. In contrast, prophylactic administration of oral anti-HBV agents plus hepatitis B immunoglobulin preceding the liver transplant aborts HBV recurrence in the allograft in most instances [182]. Recurrent HBV is currently limited to cases of the emergence of lamivudine-resistant strains. The treatment of established recurrent HBV remains controversial, although new hopes have emerged with the availability of adefovir and tenofovir [182].
The main experiences derived from OLT in patients with end-stage chronic hepatitis B are the following: (i) The risk of opportunistic infections in the post-transplant period is very low when HIV replication is well controlled with HAART, keeping most cases with an undetectable viral load. Furthermore, CD4 cell counts remain stable or even increase with HAART. Therefore, the use of standard immunosuppressive therapy in patients with well-controlled HIV-infection does not increase their susceptibility to opportunistic infections or malignant conditions. (ii) Cyclosporin and tacrolimus can inhibit HIV replication and mycophenolate mofetil may potentiate abacavir. The benefit of these interactions is currently being explored. (iii) There are important pharmacokinetic interactions between some antiretroviral agents, protease inhibitors and non-nucleoside reverse transcriptase inhibitors, and immunosuppressive agents, mainly cyclosporin and tacrolimus. Protease inhibitors may increase the levels of cyclosporin and tacrolimus, whereas non-nucleoside reverse transcriptase inhibitors may reduce their levels, as a result of their opposite effects over cytochrome p450. These interactions have caused some episodes of acute rejection in patients who stopped protease inhibitors while taking calcineurin inhibitors. The therapeutic drug monitoring of immunosuppressive agents is mandatory, especially when taking antiretroviral drugs [183]. (iv) Hepatotoxicity associated with HAART regimens can also be observed in liver allografts, and liver function should be closely monitored. (v) HBV recurrence may occur after OLT, but this risk has been dramatically reduced using hepatitis B immunoglobulin and lamivudine, adefovir or tenofovir. (vi) As survival in the waiting list seems to be much shorter in HIV-co-infected patients, strategies to make liver transplantation available sooner after a patient's assignment to this procedure should be underlined.
Panel summary
All HIV-infected patients with end-stage HBV-related liver disease should be considered as candidates for liver transplantation as long as they do not have advanced HIV disease. In those with severe immunodeficiency (<100 CD4 cells/μl) the control of HIV replication and immune restoration should be prioritized. The evaluation and the pre and postoperative medical management of HIV-positive candidates for OLT must include an interdisciplinary team composed of hepatologists, infectious diseases specialists, surgeons, psychologists, and social workers. HIV-positive candidates should have no previous history of opportunistic infections (except tuberculosis and perhaps oesophageal candidosis), and current CD4 cell counts greater than 100 cells/μl and plasma HIV-RNA levels below 200 copies/ml or with optional drugs for successful treatment in the future. Moreover, they should have abstained from the consumption of alcohol or illegal drugs for at least 6 months. The administration of oral anti-HBV agents before transplantation followed by these compounds plus hepatitis B immunoglobulin after transplantation is critical to avoid HBV recurrence in the allograft. SCORE: B2.
|
|
| |
| |
|
|
|