| |
New Hep B Drug Pradefovir: 24 week data
|
| |
| |
"Valeant Pharmaceuticals Reports Positive Data from Pradefovir Mesylate Phase 2 Study
Results from 24_Week Interim Period Show Significant Decline in HBV Viral Load with No Evidence Of Nephrotoxicity"
Valeant issued this press release.
COSTA MESA, Calif.__Valeant Pharmaceuticals International (NYSE:VRX) today reported promising 24_week interim data from a Phase 2 study of its oral anti_viral compound, pradefovir. Valeant is evaluating the safety and efficacy of pradefovir for the treatment of compensated chronic hepatitis B. Pradefovir is a pro_drug of adefovir that was licensed from Metabasis Therapeutics. Pradefovir uses Metabasis' HepDirect technology which enables higher concentrations of the drug in the liver, the primary site of hepatitis B viral (HBV) replication.
The Phase 2 study is an open_label, randomized, multiple dose study with 242 patients enrolled at 21 sites in the United States, Taiwan, Singapore and Korea. Approximately half of the patients had been previously treated ineffectively with other drugs and 70 percent of the patients were HBeAg positive. Patients that have been previously treated ineffectively are considered to be more difficult to treat. The Phase 2 study consists of five treatment groups: pradefovir _ 5, 10, 20 and 30 mg/day (QD), and Hepsera (adefovir dipivoxil) _ 10 mg/day (QD), with an overall treatment duration of 48 weeks.
The interim 24_week data indicate that pradefovir demonstrated a significant decline in HBV DNA summarized as follows:
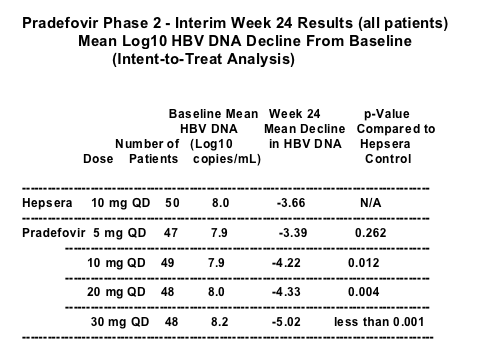
The interim results have shown no evidence of nephrotoxicity. There were no serious adverse events related to treatment. The most frequently reported adverse events were similar across all treatment groups, including Hepsera. No dose_related trends regarding safety were identified and no events resulted in a patient being withdrawn prematurely from treatment.
Timothy C. Tyson, Valeant's president and chief executive officer, said, "The strength of the Phase 2 interim data for pradefovir is very encouraging. The significant reduction in viral load, coupled with no nephrotoxicity, represents a potentially significant advantage over current therapies. Pradefovir could lead to a better treatment regimen in the rapidly growing HBV market. This development also highlights the strength of our late stage clinical portfolio, which includes four major product candidates: Viramidine(R), retigabine, Zelapar and pradefovir."
Kim D. Lamon, M.D., Ph.D., president, research and development and chief scientific officer, said, "The pradefovir interim results are very promising and better than expected, particularly in a study where approximately half of the patients previously received therapy that was ineffective. Current treatment medicines have encountered resistance after prolonged use and some have dose_limiting adverse events. If the clinical results continue to be successful, pradefovir could provide physicians with a new treatment alternative that will significantly improve patient outcomes."
The detailed Phase 2 interim results are expected to be submitted for presentation at the 56th Annual Meeting of the American Association for the Study of Liver Diseases to be held in November 2005. Patient participation in the Phase 2 trial is expected to be completed early in 2006. Valeant plans to review the interim results with the Food and Drug Administration (FDA).
About Hepatitis B
Hepatitis B is a potentially fatal disease that can lead to complications such as cirrhosis and liver cancer. Approximately 2 billion people worldwide are estimated to have hepatitis B, with 350_400 million people estimated to be chronically infected. According to a recent study, the HBV market currently represents more than $1 billion in annual sales, and is expected to grow to over $2.8 billion by 2012.
Pradefovir, Viramidine, retigabine and Zelapar are investigational compounds which have not been found by the FDA or any other regulatory agency to be safe or effective in the diagnosis, mitigation, treatment or cure of any disease or illness. They may not be sold or promoted in the United States unless and until FDA has approved their respective New Drug Applications. Similar restrictions apply in other countries.
About Valeant
Valeant Pharmaceuticals International (NYSE:VRX) is a global, publicly traded, research_based specialty pharmaceutical company that discovers, develops, manufactures and markets pharmaceutical products primarily in the areas of neurology, infectious disease and dermatology. More information about Valeant can be found at www.valeant.com.
Viramidine and Zelapar are trademarks or registered trademarks of Valeant Pharmaceuticals International or its related companies. All other trademarks are the trademarks or the registered trademarks of their respective owners.
|
|
| |
| |
|
|
|