| |
HBV, pathogenesis and natural course
|
| |
| |
....short-term goal of treatment is to ensure HBV-DNA sustained suppression....
Liver International
Volume 25 Issue 3 Page 472 - June 2005
Review Article
Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2005 update
Yun-Fan Liaw1, Nancy Leung2, Richard Guan3, George K.K. Lau4, Ismail Merican5, Geoff McCaughan6, Edward Gane7, Jia-Horng Kao8 and Masao Omata9 for the Asian-Pacific consensus update working party on chronic hepatitis B
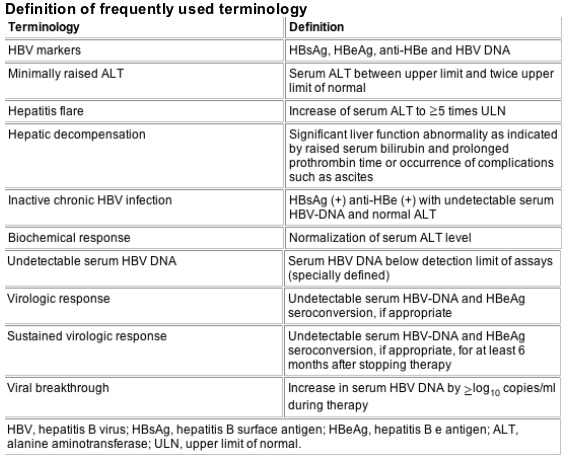
Chronic HBV infection is a serious clinical problem because of its worldwide distribution and potential adverse sequelae. It is particularly important in the Asian-Pacific region where the prevalence is high. In this part of the world, the majority of HBV infection is acquired perinatally or in early childhood. Some patients may be concurrently infected with other hepatotrophic viruses.
Previous studies revealed the presence of two replication pathways, namely episomal and integrated forms, and of reverse transcription process in HBV infection (4, 5). It has been suggested that covalently closed circular DNA plays a key role in the maintenance of chronic HBV infection (6). As HBV is not usually cytopathogenic by itself, chronic HBV infection is a dynamic state of interactions between the virus, hepatocytes and the host immune system. Accordingly, the natural course of chronic HBV infection in this geographic region can be divided into three phases: (i) immune tolerance, (ii) immune clearance and (iii) residual or inactive phase.
Immune tolerance phase is characterized by high HBV replication with little clinicopathological changes. During the immune clearance phase, hepatitis activity and even hepatitis flares with serum alanine aminotransferase (ALT) over five times upper limit of normal (ULN) may occur, and these may sometimes be complicated by hepatic decompensation. These ALT elevations and hepatitis flares are the results of the host's immune responses against HBV, such as HLA-class I antigen restricted, cytotoxic T lymphocyte (CTL)-mediated response against HBV antigen(s) expressed on hepatocytes with resultant apoptosis. Higher ALT levels, therefore, usually reflect more vigorous immune responses against HBV and more extensive hepatocyte damages. This is eventually followed by hepatitis B e antigen (HBeAg) seroconversion to its antibody (anti-HBe) and/or undetectable HBV-DNA (7). Up to 85% of HBeAg seroconversion is associated with clinical remission (inactive chronic HBV infection). However, reactivation or active hepatitis may occur because of HBeAg reversion or occurrence of HBeAg negative, HBV-DNA positive hepatitis (8, 9). The natural history of the HBeAg negative, HBV-DNA positive chronic hepatitis in the Asian-Pacific region has not been well studied, but it was demonstrated that hepatitis flares might also occur (8-10). A prospective study involving 684 patients with chronic hepatitis B showed that cirrhosis developed at an estimated annual incidence of 2.1%, and that the severity, extent, duration and frequency of hepatic lobular alterations during hepatitis flares tend to determine the disease outcome and clearance of HBV (11). One study showed that 23% and 4.4% of patients with HBeAg negative hepatitis progressed to cirrhosis and hepatocellular carcinoma (HCC), respectively during a follow-up period of 9 (1-18.4) years (8). HCC may develop at an annual incidence of 3-6% in patients with cirrhosis and might also develop, but less frequently, in non-cirrhotic background (8, 12, 13). Even in incidentally identified asymptomatic subjects with chronic HBV infection, seropositivity for HBeAg and/or HBV-DNA are risk factors for cirrhosis and HCC (9, 14, 15). Spontaneous HBsAg seroclearance may occur and usually confers excellent prognosis (16). However, HCC may still occur though at a very low rate unless cirrhosis has already developed before HBsAg seroclearance (16, 17).
Based on an intergroup divergence of 8% or more in the complete genome nucleotide sequence, HBV has been classified into at least eight genotypes (18). Each genotype has its distinct geographical and ethnic distribution (18-20). Genotypes A and D occur frequently in Africa, Europe and India, while genotypes B and C are prevalent in Asia. Genotype E is restricted to West Africa, and F is found in Central and South America. Genotype G was reported in France, Germany and the United States. Recently, the eighth genotype H has been described in Central America. Even within the Asian-Pacific region, HBV genotype distribution varies. In addition, subtypes are identified within some genotypes (21, 22); however, their clinical significance remains to be examined (23).
Several studies have shown that compared with genotype C, genotype B is associated with spontaneous HBeAg seroconversion at a younger age (24-26), less active liver disease (27-29), slower progression to cirrhosis (30) and less frequent development of HCC (18, 27, 31, 32). A study from India indicated that genotype D is more often associated with HBeAg negative chronic hepatitis B, more severe diseases and may predict the occurrence of HCC in young patients (33).
Goals of treatment
It is clear that sustained viral suppression is the key to the reduction or prevention of hepatic injury and disease progression. Therefore, the primary goal of treatment for chronic hepatitis B is to eliminate or permanently suppress HBV. This will decrease pathogenicity and infectivity, and thereby stop or reduce hepatic necroinflammation. In clinical terms, the short-term goal of treatment is to ensure HBV-DNA sustained suppression, ALT normalization and prevent the development of decompensation (initial response), to reduce hepatic necroinflammation and fibrosis during and after therapy (maintained and sustained response). The ultimate long-term goal of therapy is to prevent hepatic decompensation, to reduce or prevent progression to cirrhosis and/or HCC, and to prolong survival (durable response).
Currently available treatments
Several potentially effective agents with different mechanisms of action have entered clinical practice or clinical trials. Several nucleoside analogues (e.g. adenine arabinoside, fialuridine and lobucavir) were found to be effective, but significant toxicity precluded their further evaluation. Famciclovir is able to suppress HBV replication but phase III trials showed that it had limited efficacy. Currently, interferon-alpha (IFN-alpha), lamivudine and adefovir have been licensed globally. This paragraph did not mention it as publication timing did not permit for it but this year the oral antiviral entecavir was approved for treatment of hepatitis B. Thymosin-alpha1 has also been approved in more than 30 countries, mainly in Asia. Peginterferon alpha-2a has been granted approval in some Asian and European countries and the approval process is underway in other countries.
|
|
| |
| |
|
|
|