| |
Early Viral failure of NNRTI+ddI/TDF
|
| |
| |
"Early virological failure in treatment-naive HIV-infected adults receiving didanosine and tenofovir plus efavirenz or nevirapine"
AIDS: Volume 19(2) 28 January 2005
León, Agathe; Martinez, Esteban; Mallolas, Josep; Laguno, Montserrat; Blanco, Jose Luis; Pumarola, Tomás; Gatell, Josep María
Infectious Diseases Unit, Hospital Clinic, University of Barcelona, Spain
Abstract. A 50% rate of early virological failure associated with the selection of resistance mutations was seen in a group of 14 antiretroviral-naive adults who initiated highly active antiretroviral therapy with tenofovir and didanosine plus efavirenz or nevirapine. At month 6, the mutations detected were K65R, L74V, L100I, K103N/R/T, Y181C and G190E/Q/S. These results argue against the use of tenofovir plus didanosine in HIV-infected antiretroviral-naive adults even when the third drug is a non-nucleoside reverse transcriptase inhibitor.
The preferred highly active antiretroviral therapy consists of a backbone of two nucleoside reverse transcriptase inhibitors (NRTI) plus a protease inhibitor or a non-nucleoside reverse transcriptase inhibitor (NNRTI). When the third component is also a NRTI the virological outcome has been poorer compared with divergent combinations: slightly lower in the case of abacavir, zidovudine and lamivudine [1], or even very bad when the regimen does not include a thymidine analogue as a result of early virological failure, with the selection of resistance mutations in a high proportion of patients [2].
Until very recently the backbone of NRTI treatment has been an almost random selection of two drugs from among the available agents with the exception of zidovudine plus stavudine. The combination of tenofovir plus didanosine is very appealing (two pills in a single daily dose), but the dose of didanosine should be decreased from 400 to 250 mg a day when body weight is greater than 60 kg because of pharmacokinetic interactions, and both pills should be taken with food according to the recommendations of the manufacturers [3].
We have detected an unexpectedly high rate of early virological failure associated with the selection of resistance mutations in a group of 14 antiretroviral-naive adults who initiated antiretroviral therapy with tenofovir plus didanosine plus efavirenz or nevirapine.
From our database, we identified all antiretroviral-naive adult patients (n = 14) who initiated outside clinical trials a daily regimen consisting of tenofovir (300 mg) plus didanosine EC (250 or 200 mg if the total body weight was >= 60 or < 60 kg, respectively) plus efavirenz (600 mg; n = 10) or nevirapine (400 mg; n = 4) with food from October 2002 to March 2004, following the criteria of the treating physician. Demographic characteristics, HIV risk factors, previous HIV disease-related diagnoses, had been assessed at baseline. Clinical data including side-effects, adherence and current medications, physical examination with height and weight and laboratory parameters (blood cells, biochemical parameters, CD4 and CD8 T-cell counts, and plasma HIV-1-RNA levels; Amplicor HIV (Roche Molecular Systems, Somerville, NJ, USA), limit of detection < 200 copies/ml), had been evaluated at baseline, at month 3, and every 3 months thereafter. Virological failure was considered as a lack of response (plasma viral load drop < 2 log10 at month 3) or viral load rebound (> 200 copies/ml in two consecutives measurements separated by at least one week after the initial drop below < 200 copies/ml). Genotypic resistance tests were determined using the Viroseq HIV-1 genotyping system (Applied Biosystems, Foster City, CA, USA). Chi square and Fisher's exact tests were used for a comparison of categorical variables, and Mann-Whitney tests were used for a comparison of quantitative variables. P values less than 0.05 were considered significant.
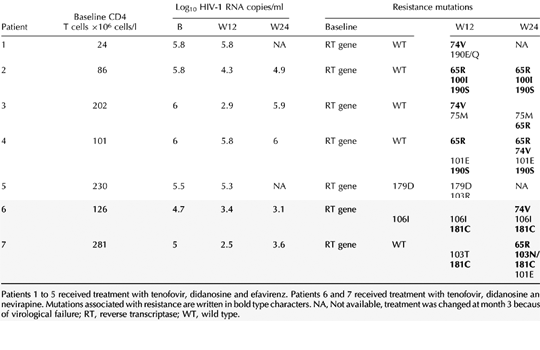
Baseline characteristics are shown in Table 1. Twelve patients completed at least 6 months of the initially scheduled therapy and the remained two patients had their regimens changed at month 3 because of virological failure. All patients had good adherence to prescribed therapy, according to physician interview. At month 3, nine out of 14 patients had a viral load drop of 2 log10 or greater and five patients (36%) had a suboptimal response. The change from baseline in log10 HIV-1-RNA copies/ml ranged from -3.7 to 0, with a median of -2.5 log10, and three of these early failing patients had only a 0.2 log10 or less plasma viral load decrease. At month 6, two patients (nos. 3 and 7; Table 2) developed a viral load rebound after an initial response at month 3. Therefore, the overall virological failure rate at month 6 was 50% (seven out of 14 patients). The only clinical characteristic that we have identified distinguishing patients who responded from those who did not both in the univariate and multivariate analysis was a greater body mass index (BMI) (P = 0.003; Table 1). No differences were found concerning the total body weight, the baseline HIV-1-RNA level, the baseline CD4 T-cell count and the number of previous AIDS-defining events (Table 1, below).
A genotype resistance test was performed in all failing patients at baseline, and at months 3 and 6. No mutations in the reverse transcriptase (RT) gene associated with resistance [4], were observed at baseline (Table 2). Concerning the protease gene, four patients had a single mutation (V77I or L10I) potentially associated with resistance to protease inhibitors, as well as other polymorphisms (L63P, M36I, I93L) frequently observed in the genotypes of antiretroviral-naive patients [4]. At month 6, resistance tests showed the L74V plus K65R mutations in four out of seven patients (57%), G190S plus Y181C in two (29%), and the L100I or K103N in one patient each (14%). Patient 5 selected the 103R mutation at month 3, which combined with the V179D mutation already present at baseline has been associated with resistance to NNRTI [5].
High rates of early virological failure have been reported with several regimens that include the combination of tenofovir plus lamivudine plus either didanosine or abacavir in antiretroviral-naive patients [2]. Virological failures in those studies could be explained by or were associated with a high rate of the selection of K65R and M184V mutations, or both [6]. Although the exact mechanism explaining this high rate of early virological failure remains unknown, the fact that all three drugs belong to the same family of NRTI might be the reason for a low potency or some antagonistic intracellular interaction. Alternatively, the low genetic barrier for resistance might also play a role.
Our results suggest that the combination of didanosine plus tenofovir even when the third drug is an NNRTI may also lead to similar high rates of virological failure as those reported with the triple NRTI regimens. A similar suboptimal response with the selection of resistance mutations has also been reported in a small randomized trial [7]. This is in contrast with the high efficacy shown by other combinations of two NRTI plus a NNRTI, which represent the standard of first-line therapy in most current guidelines. The co-administration of didanosine EC plus tenofovir, furthermore, has been associated with a decrease or with a lack of a sustained increase in CD4 T cells [8] and with a higher rate of pancreatitis when compared with regimens containing didanosine without tenofovir or tenofovir without didanosine [9]. On the basis of these results, the use of an NNRTI in combination with tenofovir and didanosine as an initial therapy in antiretroviral-naive patients cannot be recommended. The mechanisms involved in this early virological failure remain to be elucidated.
Irrespective of how appealing a potential combination of drugs might be, our data strongly support acting with caution while awaiting the results of sufficiently powered randomized trials to confirm the safety and efficacy of any combination of antiretroviral agents before jumping into the clinical arena.
REFERENCES
1 Gulick RM, Ribaudo HJ, Shimuka CM, Lustgarten S, Meyer WA, Klingman K, et al. Triple-nucleoside regimens versus efavirenz-containing regimens for the initial treatment of HIV-1 infection. N Engl J Med 2004; 350:1850-1861.
2 Wainberg MA, Turner D. Resistance issues with new nucleoside/nucleotide backbone options. J Acquir Immune Defic Syndr 2004; 37:S36-S43.
3 Kaul S, Damle B, Bassi K, Xie J, Gale J, Ryan K, et al. Pharmacokinetic evaluation of reduced doses of didanosine enteric coated capsules (didanosine-EC) in combination with tenofovir disoproxil fumarate (TDF) and food for a once-daily antiretroviral regimen. Presented at: 4th International Workshop on Clinical Pharmacology of HIV Therapy. Cannes, March 2003 [Abstract 8.1].
4 D'Aquila RT, Schapiro JM, Brun-Vézinet F, Clotet B, Conway B, Demeter LM, et al. Drug resistance mutations in HIV-1. Topics HIV Med 2003; 11:92-96.
5 Petropoulos CJ. High-level resistance to HIV-1 non-nucleoside reverse transcriptase inhibitors (NNRTIs) in the absence of known resistance mutations. Presented at: 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy. Chicago, September 2003 [Abstract H-451].
6 Jemsek J, Hutcherson P, Harper E. Poor virologic responses and early emergence of resistance in treatment naive, HIV-infected patients receiving a once daily triple nucleoside regimen of didanosine, lamivudine, and tenofovir DF. Presented at: 11th Conference on Retroviruses and Opportunistic Infections. San Francisco, February 2004 [Abstract 51].
7 Podzamczer D, Ferrer E, Gatell JM, Niubo J, Dalmau D, Leon A, et al. Early virologic failure and occurrence of resistance in naive patients receiving tenofovir, didanosine and efavirenz. Presented at: XIIIth International HIV Drug Resistance Workshop. Tenerife, June 2004 [Abstract 156].
8 Negredo E, Moltó J, Burger D, Viciana P, Ribera E, Paredes R, et al. Unexpected CD4 cell-count decline in patients receiving didanosine and tenofovir-based regimens despite undetectable viral load. AIDS 2004; 18:459-463.
9 Martinez E, Milinkovic A, De Lazzari E, Ravasi G, Blanco JL, Larrousse M, et al. Pancreatic toxic effects associated with co-administration of didanosine and tenofovir in HIV-infected adults. Lancet 2004; 364:8-10.
Table 1
|
|
| |
| |
 |
|
| |
| |
|
|
| |
| |
 |
|
| |
| |
|
|
| |
| |
|
|
|