| |
Managing Cardiovascular Risk in Patients With HIV Infection
|
| |
| |
JAIDS Journal of Acquired Immune Deficiency Syndromes: Volume 38(2) 1 February 2005
Stein, James H MD
From the Section of Cardiovascular Medicine, University of Wisconsin Medical School, Madison, WI.
Received for publication May 19, 2004; accepted September 28, 2004.
Summary:
Highly active antiretroviral therapy (HAART) improves the survival of patients with HIV infection; however, several observational studies have described associations between HIV infection, HAART, and cardiovascular disease. Important limitations of these studies included a low incidence of cardiovascular events, short duration of HAART exposure, and retrospective design. Nevertheless, the weight of evidence from observational and surrogate end point studies suggests that the dyslipidemia and other metabolic changes that are common in patients with HIV infection and those using HAART may be associated with increased cardiovascular risk. The Infectious Disease Society of America/Adults AIDS Clinical Trials Group guidelines for the evaluation and management of dyslipidemia recommend target lipid levels and treatment of dyslipidemia in patients with HIV infection. Although practitioners should consider dyslipidemia and cardiovascular risk when making plans for initiating or altering HAART therapy, maintaining viremic control should be the overriding factor, because short-term absolute rates of cardiovascular disease are significantly lower than death rates from AIDS in inadequately suppressed patients. This article reviews the cardiovascular risks in patients receiving HAART and discusses the implementation of the new guidelines.
In 1986, when the Centers for Disease Control and Prevention provided the first working definition for AIDS, the median survival after HIV infection was approximately 10 years and the median survival after a diagnosis of AIDS was approximately 2 to 4 years.1 In 1993, AIDS became the leading cause of death for Americans between the ages of 25 and 44 years old. With the release of saquinavir, the first HIV protease inhibitor (PI), in 1995 and the subsequent approval of 3 more PIs in the next 18 months, the AIDS-related death rate in the United States decreased for the first time in 1996.1 Currently, more than 85% of patients with HIV infection survive more than 10 years.1 The introduction of highly active antiretroviral therapy (HAART) has changed the goal of therapy for HIV infection from simply keeping the patient alive to one that balances toxicity with benefits, tries to maximize compliance, and focuses on long-term consequences of HIV infection and its therapy.
HAART AND CARDIOVASCULAR RISK
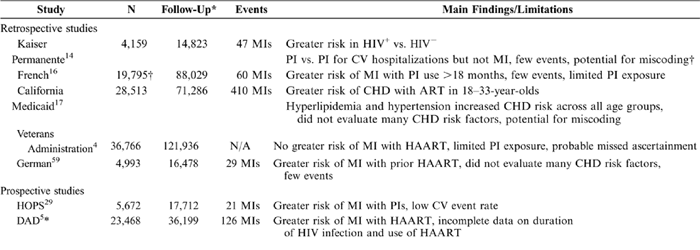
The absolute risk associated with uncontrolled AIDS clearly is much greater than that associated with cardiovascular disease. This was observed in a surveillance study of 1255 HIV-positive patients, in which mortality declined from 21.9 per 100 person-years in 1994 to 3.7 per 100 person-years by mid-1997.2 Combination therapy was associated with the greatest reductions in morbidity and mortality. These results were consistent with findings that HIV-infected patients who initiated antiretroviral therapy when CD4+ cell counts were 201 to 350 cells/mm3 had mortality rates of 15.4 per 1000 person-years compared with 56.4 per 1000 person-years in those who delayed therapy.3 A retrospective study of cardiovascular and cerebrovascular risk among 36,766 HIV-infected patients at Veterans Affairs (VA) hospitals (January 1993-June 2001) found that the total mortality rate decreased from 21.3 to 5.0 deaths per 100 patient-years.4 This overall decline in mortality was attributable to antiretroviral therapy, highlighting the concept that the need for effective viral suppression is more pressing than concerns about potential cardiovascular risks. Although it is now clear that HAART improves survival of patients with HIV infection, there is increasing epidemiologic overlap between patients with HIV infection and those at risk for coronary artery disease. Common risk factors include increasing age, a high prevalence of tobacco use, and a disproportionately increased number of African-American and Hispanic persons.5,6 Furthermore, risk factors for coronary artery disease such as hyperlipidemia, hyperglycemia, and central obesity develop in more than 60% of patients receiving HAART, which has raised concerns that cardiovascular disease may become an important complication of HIV infection.7-9 Before the advent of HAART, a case series of 8 HIV-positive patients (23-32 years old) with no familial risk factors found striking coronary lesions in these patients postmortem, suggesting that HIV infection itself was associated with these lesions.10 Similarly, a matched-control study of 30 HIV-positive patients found that atherosclerosis was more common in HIV-positive patients than in controls.11 Case reports of premature coronary heart disease (CHD) in patients receiving HAART started to appear in the literature shortly after the emergence of HAART.12,13 In the past few years, studies have attempted to clarify the associations between HIV infection, HAART, and cardiovascular disease. These studies have had major limitations, including a low incidence of reported adverse cardiovascular events; short duration of exposure to HAART; retrospective study design with nonsystematic assessment of CHD risk factors, lack of medication compliance data, and the limitations inherent in ascertainment of case status by discharge and diagnostic codes. What follows is a summary of several recently published studies that have provided useful, albeit imperfect, data regarding HIV, HAART, and cardiovascular risk (Table 1).
|
|
| |
| |
 |
|
| |
| |
RETROSPECTIVE STUDIES
The Kaiser Permanente Registry study ascertained data from discharge diagnoses in members of the Kaiser Permanente Northern California Health Maintenance Organization (see Table 1). This data set identified 4159 HIV-positive men aged 35 to 64 years old (median = 42 years) from January 1996 through December 31, 2003.14,15 In patients with HIV infection, the age-adjusted CHD event rate was 6.3 per 1000 patient-years (P < 0.001) compared with 3.7 for HIV-negative controls (P = 0.006); however, the age-adjusted rate of myocardial infarction (MI) in patients receiving PIs was not statistically higher than in patients not receiving PIs (4.0 vs. 3.4 per 1000 patient-years). The median exposure to these medical therapies only was 1.2 and 4.2 years, respectively. Of note, in a recently reported update from this data set, a statistically significant relation between CHD hospitalization and PI exposure was identified, in which the age-adjusted relative risk per 2 years of PI exposure was 1.17 (P = 0.01). The relative risk for MI, however, remained nonsignificant.15
The French Hospital Database on HIV reported a relationship between the duration of PI exposure and the occurrence of MI among 34,976 men during 88,029 patient-years of follow-up.16 Sixty men, including 49 who had received a PI, had an MI. Exposure to a PI was associated with a relative hazard rate for MI of 2.56 (95% confidence interval [CI]: 1.03-6.34). The standardized morbidity ratio of men exposed to PIs for <18 months, relative to the French general male population, was 0.8 (range: 0.5-1.3) but increased to 2.9 (range: 1.5-5.0) for men exposed to PIs for >=30 months.
In the California Medicaid (Medi-Cal) database of 28,513 HIV-infected persons, the incidence of CHD among men (18-33 years old, relative risk: 2.16-6.76; P < 0.0001) and women (18-44 years old, relative risk: 1.53-2.47; P < 0.011) was significantly higher than among individuals without HIV infection.17 In covariate-adjusted models, the relative risk for developing CHD in those receiving HAART was 2.06 (P < 0.001) for those <33 years old. There was no association between HAART exposure and CHD in other age groups.
In a retrospective study of data from the VA Quality Enhancement Database for HIV, 36,766 HIV-infected patients (98% men) were followed for an average of 40 months (January 1993-June 2001).4 The incidence of cardiovascular or cerebrovascular events did not change and was not significantly higher among patients taking PIs than among those not taking these medications. Regression analyses showed no relationship between use of any HIV therapy and the incidence of cardiovascular or cerebrovascular events, although use of antiretroviral therapy was associated with a reduced risk of death. The median duration of exposure to PIs was only 16 months. The true cardiovascular disease rate may have been underestimated, because many patients with acute coronary syndromes may not have been admitted to VA hospitals and cardiovascular services at VA hospitals probably did not remain constant during the period of ascertainment.
SURROGATE END POINT STUDIES
Limitations similar to those of the retrospective studies also apply to the multitude of surrogate end point studies that have been published looking at anatomic measures of atherosclerosis, including carotid intima-media thickness and coronary artery calcium.18-22 Still, because a major limitation of clinical research investigating cardiovascular events in patients with HIV infection is the low absolute event rate, use of validated surrogate markers in well-designed studies is appropriate, since surrogate markers or their changes occur more frequently and can improve statistical power. Most but not all of these studies have in fact shown an increased prevalence of subclinical atherosclerosis in patients receiving HAART.18-24 With 1 exception, several studies also demonstrated that the use of HAART is associated with impaired endothelial function, a marker of future cardiovascular risk.25-28 One of the larger (n = 168) longer term (6 months) studies found that a significantly higher percentage of HIV-infected individuals had not exhibited atherosclerotic plaques (55% vs. 38%; P = 0.02) however they were associated with classic cardiovascular risk factors such as smoking and hyperlipidemia rather than with the use of PIs per se.19 Several of these studies concluded that the abnormalities in the surrogate cardiovascular marker were associated with increased prevalence of traditional cardiovascular risk factors such as dyslipidemia and insulin resistance.18,21-23,28 Although this evidence is indirect, it points to the clinical relevance of using surrogate markers to predict cardiovascular risk in patients with HIV infection.
PROSPECTIVE STUDIES
Prospective observational cohorts with more systematic assessment of CHD risk factors and validation of outcomes provide more insight into the epidemiology of CHD in the HAART era and are more generalizable. Generally speaking, results from well-controlled prospective studies should be given more weight than results of retrospective studies. In the HIV Outpatient Study (HOPS) of 5672 HIV-positive patients (mean age = 42.6 years) from 9 US clinics, 21 MIs occurred during 17,712 patient-years of follow-up (January 1993-January 2002) (see Table 1).29 MIs occurred in 19 patients on PIs (1.42 per 10,000 patient-years) and in 2 patients not on PIs (0.46 per 10,000 patient-years, odds ratio = 7.1, 95% CI: 1.6-44.3). The incidence of MI in this cohort increased in a linear fashion (P = 0.0125). In multivariate Cox proportional hazards models adjusted for smoking, sex, age, diabetes mellitus, hyperlipidemia, and hypertension, the hazard ratio was 6.5 (95% CI: 0.49-47.8), suggesting that the relationship between PI use and MI no longer was statistically significant after adjustment for covariates. The difference in MI rates between groups could be a result of chance or could be associated more strongly with other covariates that were not evenly distributed between the groups. Also, the low MI rate may have reduced the power to determine a true association. Results of extended follow-up were consistent with the original findings.30 It is important to note that this study relied heavily on outpatient reporting of events that primarily occurred in hospitalized patients. In addition, the number of visits required to qualify for study inclusion and the number of MIs were low. These factors may have led to an underreporting of events.
The Data Collection of Adverse Events Study (DAD) was a prospective observational study of 23,468 patients (median age = 39 years) from 11 cohorts in Europe, Australia, and the United States.31 Many of the subjects had received HAART for several years before entry, such that the exact duration of HAART exposure before 1999 was not clear and almost certainly varied from zero to more than 6 years of previous treatment. During this study, 126 MIs occurred, for an incidence rate of 3.5 per 1000 patient-years. The incidence of MI increased with increasing exposure to HAART, and the adjusted risk rate per year of exposure ranged from 0.32 for no HAART use to 2.93 for >=6 years of HAART use. Significant predictors of incident MI included a history of cardiovascular disease (relative risk = 5.8; P < 0.001), current or former smoking (relative risk = 2.17; P < 0.007), use of HAART (relative risk = 1.26; P < 0.001), age (relative risk = 1.38; P < 0.001), and male sex (relative risk = 1.99; P < 0.04). Body mass index, mode of HIV infection, race, and family history of CHD did not predict incident MI. The association between HAART and MI decreased with inclusion of total cholesterol and triglycerides levels but did not change with inclusion of diabetes mellitus, hypertension, the presence of lipodystrophy, and markers of HIV infection. This suggests that during the first 4 to 6 years of HAART therapy, there was approximately a 26% increase in the relative risk of having an MI. Part of the increased risk is associated with the development of dyslipidemia, but the absolute rate of MI is low in light of the benefits of antiretroviral therapy. Additional prospective studies evaluating cardiovascular risks of HIV infection and its various treatments clearly are needed, with appropriate design and statistical consideration of the effects of treatment of cardiovascular risk factors that develop while on HAART and the effects of changes in antiretroviral regimens. Prospective treatment of risk factors and alterations (including advances) in HAART represent formidable challenges to understanding the predictors of cardiovascular disease in patients with HIV infection.
POTENTIAL MECHANISMS OF HIV-ASSOCIATED DYSLIPIDEMIA TOP
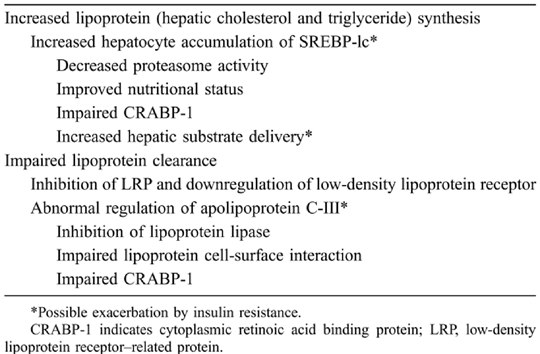
Several potential mechanisms by which PIs could lead to dyslipidemia have been proposed (Table 2). Although a discussion of these mechanisms is beyond the scope of this article, it can generally be stated that proposed mechanisms include those affecting lipoprotein production and clearance.32,33 Increased hepatic cholesterol and triglyceride synthesis could occur as a result of hepatocyte accumulation or increased hepatic substrate delivery, factors exacerbated by insulin resistance. Inhibition of proteasome activity may lead to increased levels of sterol-regulatory element-binding protein (SREBP) and apolipoprotein B-100 (apoB-100) in hepatocytes. Proposed mechanisms include suppression of breakdown of SREBP and of proteasomal breakdown of apoB-100 as well as altered function of lipoprotein lipase. Increased hepatocyte accumulation of SREBP-1c may be a result of several factors, as detailed in Table 2. All these factors may contribute to increased very low-density lipoprotein (VLDL) and, possibly, low-density lipoprotein (LDL). Impaired lipoprotein clearance could be caused by inhibition of the hepatic LDL receptor-related protein or by abnormal regulation of apolipoprotein C-III, a plasma inhibitor of VLDL lipolysis. Although in vitro and animal models have elaborated some of these mechanisms, there are few supporting clinical studies.32 There is some evidence that different PIs may have different effects on fat metabolism.33 None of the mechanisms alone explains all the metabolic abnormalities observed in HIV-infected patients receiving HAART, pointing to a multifactorial process and the need for further research. Similarly, although it is known that nucleoside and nonnucleoside reverse transcriptase inhibitors also may affect lipid metabolism, the mechanisms are not yet known.5
|
|
| |
| |
 |
|
| |
| |
GUIDELINES FOR EVALUATING AND MANAGING DYSLIPIDEMIA IN PATIENTS RECEIVING HAART
Because the weight of evidence from prospective, observational, and surrogate end point studies suggests that HAART may be associated with an increased risk of cardiovascular events and that may be related in part to dyslipidemia, the Infectious Disease Society of America (IDSA) and Adult AIDS Clinical Trials Group (AACTG) updated their guidelines for the evaluation and management of dyslipidemia in HIV-infected adults receiving antiretroviral therapy.34 These guidelines are based on those provided by the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) guidelines, in which the intensity of risk reduction therapy is adjusted to the patient's risk of developing cardiovascular disease.35
Evaluation of Risk
Evaluation of dyslipidemia begins with obtaining a lipid panel after a minimum of 8 hours, and preferably 12 hours, of fasting (Fig. 1). The screening lipid panel should include measurement of total cholesterol, high-density lipoprotein cholesterol (HDL-C), and triglycerides levels, from which low-density lipoprotein cholesterol (LDL-C) and non-HDL-C levels should be calculated. The lipid panel should be repeated within 3 to 6 months after initiation of HAART and then yearly unless lipid abnormalities are detected or interventions are initiated. For patients with triglycerides >200 mg/dL, a lipid panel should be repeated within 1 to 2 months after starting HAART.
The patient's level of cardiovascular risk determines the target lipid levels and the aggressiveness of lipid-lowering therapy. High-risk patients include those with established CHD or those considered a coronary risk equivalent; the latter category includes patients with cerebrovascular disease, peripheral vascular disease, diabetes mellitus, or multiple risk factors that predict a 10-year risk of cardiac death or MI of >20%, as discussed later in this article (see Fig. 1). After identifying high-risk patients, the remaining patients are categorized by counting the presence of risk factors that modify lipid-lowering therapy. These risk factors include smoking cigarettes, hypertension (blood pressure >=140/90 mm Hg or treatment with an antihypertensive medication), low HDL-C (<40 mg/dL), a family history of premature CHD (in a male first-degree relative <55 years old or a female first-degree relative <65 years old), and increased age (man >=45 years old or woman >55 years old). Of note, elevated HDL-C >60 mg/dL counts as a negative risk factor and, for the purpose of counting risk factors, mitigates the presence of 1 risk factor. If 2 or more risk factors are present, the NCEP ATP III and IDSA/AACTG guidelines recommend calculating the patient's absolute 10-year risk of cardiac death or MI using the Framingham risk factor calculator, which can be found at http://www.nhlbi.nih.gov/guidelines/cholesterol/index.htm .34,35
Framingham risk calculation is easy and accurately relates CHD risk factors to an individual patient's CHD risk. Framingham estimates should be thought of only as providing a starting point for risk assessment, because predisposing risk factors such as obesity, physical activity, and socioeconomic status and conditional risk factors such as lipoprotein(a) excess, lipoprotein particle sizes, and genetic susceptibility are not included in the assessment.36,37 It is especially important for practitioners treating patients with HIV infection to remember that these prediction algorithms only address short-term (10-year) risk, which may not be an appropriate time frame for young and middle-aged adults. In HIV-infected individuals, this algorithm does not account for any possible risks that chronic HIV infection may contribute to cardiovascular risk. Furthermore, this algorithm does not account for duration of exposure to risk factors. A patient who smoked heavily but quit more than a year before evaluation is assessed the same as a lifelong nonsmoker. Conversely, a lipid abnormality that may not have been present before HIV infection or the use of HAART may not confer the same level of risk as a genetic dyslipidemia. For many patients with HIV who are tolerating their medical regimen, are physically well, and have had excellent long-term viral suppression, the lifetime risk of CHD may be a more appropriate consideration.38 Nevertheless, Framingham estimates of 10-year risk are a good starting point for patient evaluation and management.
Determining Target Lipid Levels
After 10-year coronary risk has been estimated, lipid targets should be determined (Table 3). Except for patients with triglycerides >500 mg/dL, in whom the primary goal of therapy is to reduce triglycerides levels and prevent pancreatitis, the primary target is reduction of LDL-C (see Fig. 1). For patients with zero to 1 risk factor (who generally have a 10-year CHD risk of <10%), the target LDL-C is 160 mg/dL. For patients with 2 or more risk factors, the target LDL-C is 130 mg/dL unless they have a CHD risk equivalent condition. In patients with CHD or CHD equivalent risk, the target LDL-C is 100 mg/dL. When patients have triglycerides >200 mg/dL, the cholesterol content of triglyceride-rich lipoproteins is increased and the estimated LDL-C underestimates the number of atherogenic particles. Non-HDL-C (calculated as total cholesterol minus HDL-C) becomes a secondary target of therapy. Non-HDL-C contains all the cholesterol that is carried by the lipoproteins that are currently considered to be atherogenic, independently predicts CHD risk, and makes management of triglycerides and mixed lipid disorders less confusing. It also has the advantage of being measurable under nonfasting conditions. The non-HDL-C goal is simply the LDL-C goal plus 30 mg/dL, representing the usual cholesterol concentration carried in VLDLs (see Table 3).
It is important to consider the non-HDL-C level (or its mathematical equivalent, the total cholesterol/HDL-C ratio) when evaluating lipids in patients with HIV infection, because such patients frequently have hypertriglyceridemia or combined lipid disorders. In patients with hypertriglyceridemia, the LDL-C concentration underestimates the number of LDL particles and the overall atherogenic lipoprotein burden, because increased levels of chylomicrons, large VLDLs, and remnant lipoproteins are commonly present.35,39,40 Its particular relevance was emphasized in a recent study evaluating the impact of HIV infection and HAART on lipids in men.41 The Multicenter AIDS Cohort Study was a prospective study of homosexual and bisexual men that stored serum samples obtained between 1984 and 2002. In 50 of 517 HIV serum converters, significant declines in total cholesterol, HDL-C, and LDL-C were observed after HIV infection. Significant increases in total cholesterol and LDL-C values were observed after HAART initiation. The median HDL-C remained less than baseline values throughout follow-up in this study. Of note, the median triglycerides level was 225 mg/dL (interquartile range: 147-331 mg/dL) after the third post-HAART visit. The authors concluded that initiation of HAART increased total cholesterol and LDL-C and that this may return to preinfection serum lipid levels after accounting for expected age-related changes. This analysis, however, may have underestimated the increased risk associated with the post-HAART lipid values as compared with pre-seroconversion levels. Although the non-HDL-C levels were not reported, based on the median values that were reported, there was an approximately 28% increase in non-HDL-C and a 36% increase in the total cholesterol HDL-C ratio.41 These values could be associated with up to a 50% relative increase in the risk of MI or cardiac death. Furthermore, the data were collected without regard to fasting status, such that changes in triglycerides were not accounted for and were greater than what would be expected. An evaluation of the lipid data using non-HDL-C suggests that cardiovascular risk is increased and is higher than preseroconversion levels in patients receiving HAART. When comparing the effects of different antiretroviral regimens on plasma lipids, non-HDL-C (or the total cholesterol/HDL-C ratio) should be considered, because this takes into account effects that HIV therapy may have on total cholesterol and HDL-C.
Treatment of Dyslipidemia
In general, lifestyle changes and pharmacologic therapy are initiated if lipid levels are higher than their targets (Fig. 2).34 For patients with CHD or a coronary risk equivalent, the target LDL-C is <100 mg/dL and drug therapy is initiated when LDL-C is >130 mg/dL. Drug therapy is optional when LDL-C is between 100 and 130 mg/dL. For patients with 2 or more risk factors, the LDL-C target is 130 mg/dL. For those with a 10-year risk of 10% to 20%, drug therapy is initiated when LDL-C is >=130 mg/dL. For those with a 10-year risk of <10%, drug therapy is initiated when the LDL-C level is >=160 mg/dL. Lifestyle changes are indicated at lower LDL-C values. For individuals with none or 1 risk factor, the LDL-C target is <160 mg/dL. Drug therapy is initiated when LDL-C is >=190 mg/dL. It is considered optional when between 160 and 190 mg/dL. For patients with triglycerides >200 mg/dL, after the LDL-C target is achieved, the non-HDL-C targets are <130 mg/dL (CHD or coronary risk equivalent), <160 mg/dL (>=2 risk factors), and <190 mg/dL (0-1 risk factor).
Treatment of HAART-associated dyslipidemia includes 3 broad categories and interventions, including altering the approach to HAART, lifestyle changes, and lipid-lowering medications (see Fig. 2). There has been some concern that certain PIs may be more associated with dyslipidemia.31,34,42,43 It is possible to substitute one PI for another or to switch to a non-PI-containing regimen when PI therapy leads to dyslipidemia.34,43 The effects of switching antiretroviral regimens have been reviewed elsewhere.34,44 Although the prescribed HAART regimen can be altered, maintaining virologic and immunologic control is the overriding consideration.
Lifestyle changes are critical, and there are excellent data suggesting that exercise and dietary changes can improve lipid levels and reduce cardiovascular risk. Although a detailed discussion of exercise and dietary prescriptions is beyond the scope of this article, patients with HIV infection should exercise for a minimum of 30 min/d 5 times per week, with a goal of 60 min/d 5 to 7 times per week. This will improve cardiovascular fitness and help with weight loss, if appropriate.45,46 Patients who are overweight should also restrict calories to achieve their ideal body weight. Nutrient composition changes that have been associated with improved cardiovascular outcomes include substitution of nonhydrogenated unsaturated fats for saturated fats and trans-fats; increased intake of É÷-3 fats from fish, fish oil, or plants; and eating a diet that is high in fruits, vegetables, nuts, and whole grains but low in refined grains.47 These changes are similar to those outlined in the Mediterranean and Indo-Mediterranean diets, which have been proven to reduce cardiovascular events and cancer.48-50 Similar diets have been proven to prevent the development of diabetes mellitus.51,52
Lipid-Lowering Agents
When pharmacologic therapy is needed, initial choices are based on the predominant lipid abnormality. For patients with hypertriglyceridemia (>=500 mg/dL), a fibrate such as gemfibrozil or fenofibrate is preferred, with prescription niacin or fish oil capsules as an alternative. For patients with triglycerides <500 mg/dL, LDL-C is the primary target. If LDL-C is greater than the target level (or non-HDL-C when triglycerides are between 200 and 500 mg/dL), a statin is the first choice, with fibrates or prescription niacin as alternatives in patients with mixed disorders. The preferred statins in the IDSA/AACTG guidelines are pravastatin, atorvastatin, and fluvastatin because they have been prospectively studied in this population of patients without reports of significant toxicity. Unfortunately, the lipid-lowering effects of statins in patients receiving PIs have not frequently led to achievement of target goals. Pravastatin and fluvastatin have the least potential for drug interaction but are relatively less potent lipid-lowering agents.34 Pravastatin levels have been shown to decrease in combination with saquinavir/ritonavir, but more potent statins such as atorvastatin and simvastatin have been associated with significantly increased serum levels. Simvastatin and lovastatin should not be used in patients taking PIs; however, atorvastatin and the combination of pravastatin and fenofibrate can be used cautiously in patients on PIs.34 Studies investigating the safety and efficacy of rosuvastatin are expected within the next few years. It is important to note that many PIs interact with the cytochrome P450 system and may affect the potential toxicities of other medications.33 Statins and fibrates may cause changes in hepatic transaminase levels. Routine laboratory monitoring, including baseline hepatic transaminase levels and repeat assessment after 4 to 6 weeks, followed by per-product labeling, should be instituted for all patients receiving lipid-lowering therapy so that lipid levels and toxicity can be monitored. Routine testing of muscle enzymes is not recommended.
Second-line agents include ezetimibe for patients with increased LDL-C levels; however, studies in patients with HIV infection have not been reported. Similarly, fish oils are useful in patients with hypertriglyceridemia but have not yet been studied in detail in HIV-infected patients. Prescription niacin is useful as a second-line therapy for all dyslipidemias and is currently under study by the AACTG. Niacin and ezetimibe also require monitoring of hepatic transaminases. Niacin may cause hyperglycemia and hyperuremia. Combination therapy with a statin is needed in many patients. When fibrates and statins are used together, hepatotoxicity and muscle toxicity may be observed. When statins are combined with a fibrate, fenofibrate is preferred because it has been studied in patients with PI-associated dyslipidemia and it interferes less with statin metabolism.53,54
Beyond the IDSA/AACTG Guidelines
The results of several recent studies in patients without HIV infection have led many cardiologists to treat lipids to more aggressive targets than suggested by the IDSA/AACTG guidelines, especially individuals with CHD or at intermediate to high risk of CHD and its complications.55-58 Although a review of these studies is beyond the scope of this article, readers are encouraged to review these data and the recent update to NCEP ATP III guidelines published in July 2004.59 Because the IDSA/AACTG guidelines draw heavily on the NCEP ATP III guidelines, their recommendations should apply to patients with HIV infection. The results of these studies suggest that an LDL-C target of 70 mg/dL may be appropriate in certain high-risk patients, such as those with a baseline LDL-C <100 mg/dL, a recent acute coronary syndrome, established cardiovascular disease, and multiple or suboptimally treated risk factors (eg, diabetes mellitus, continued cigarette smoking, metabolic syndrome), and some have recommended this goal for patients with chronic kidney disease.55-59 Certain intermediate-risk patients (those with 2 or more risk factors and a 10-year risk of 10%-20%) may benefit from an LDL-C target of 100 mg/dL. These patients include those with suboptimally treated risk factors (eg, continued smoking), metabolic syndrome, or subclinical coronary artery disease. Appropriate non-HDL-C targets would be 30 mg/dL higher than corresponding LDL-C targets.59 The roles of advanced lipoprotein testing, measurement of C-reactive protein using highly sensitive assays, use of more potent lipid-lowering agents, and combination lipid-lowering therapy in patients with HIV infection are active areas of investigation. The benefit of aggressive lipid management must be balanced with the risk of additional medications, potential for drug interactions, additional pill burden, compromise in antiretroviral adherence, and potential compromise of optimal HIV control.
CONCLUSIONS
Maintaining viremic control should be the overriding concern in HIV-positive patients, because short-term rates of cardiovascular disease remain low and are significantly lower than death rates from AIDS in patients with inadequate viral suppression. The weight of evidence from observational and surrogate end point studies suggests that HIV infection and HAART may be associated with an increased risk of future cardiovascular events. Part of this increased risk may be related to the dyslipidemia and other metabolic changes that are associated with HIV infection and HAART. The IDSA/AACTG guidelines for the evaluation and management of dyslipidemia draw heavily on the NCEP ATP III guidelines by recommending target levels for LDL-C, non-HDL-C, and treatment of lipid disorders based on CHD risk. Switching HAART can be considered, although maintaining viremic control should be the main goal for therapy. Appropriate lifestyle measures, including dietary changes, exercise, and smoking cessation, are critical. Pharmacologic therapy usually includes treatment with statins, frequently in combination with fenofibrate. In the next few years, the results of several studies looking at the efficacy of other lipid-lowering agents such as niacin, ezetimibe, fish oil capsules, and newer statins should be published, as should the results of studies using surrogate end points such as endothelial function and carotid intima-media thickness, which will assess the natural history of cardiovascular disease in patients with HIV on different therapeutic regimens.
REFERENCES
1. Sepkowitz K. AIDS-the first 20 years. N Engl J Med. 2001;344:1764-1772.
2. Palella F, Delaney K, Moorman A, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853-860.
3. Palella F, Deloria-Knoll M, Chmiel J, et al. Survival benefit of initiating antiretroviral therapy in HIV-infected persons in different CD4+ cell strata. Ann Intern Med. 2003;138:620-626.
4. Bozzette S, Ake C, Tam H, et al. Cardiovascular and cerebrovascular events in patients treated for human immunodeficiency virus infection. N Engl J Med. 2003;348:702-710.
5. Friis-Moller N, Weber R, Reiss P, et al. Cardiovascular disease risk factors in HIV patients-association with antiretroviral therapy. Results from the DAD study. AIDS. 2003;17:1179-1193.
6. Shelton D. Changing demographics in HIV infections. 2000. Available at: http://www.ama-assn.org/amednews/2000/10/23/hisa1023.htm .
7. Carr A, Samaras K, Burton S, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12(Suppl):F51-F58.
8. Dube M, Sattler F. Metabolic complications of antiretroviral therapies. AIDS Clin Care. 1998;10:41-44.
9. SoRelle R. Vascular and lipid syndromes in selected HIV-infected patients. Circulation. 1998;98:829-830.
10. Tabib A, Greenland T, Mercier I, et al. Coronary lesions in young HIV-positive subjects at necropsy. Lancet. 1992;340:730.
11. Constans J, Marchand J-M, Conn C, et al. Asymptomatic atherosclerosis in HIV-positive patients: a case-control ultrasound study. Ann Med. 1995;27:683-685.
12. Gallet B, Pulik M, Genet P, et al. Vascular complications associated with use of HIV protease inhibitors. Lancet. 1998;351:1958-1959.
13. Henry K, Melroe H, Huebsch J, et al. Severe premature coronary artery disease with protease inhibitors. Lancet. 1998;351:1328.
14. Klein D, Hurley L, Quesenberry J, et al. Do protease inhibitors increase the risk for coronary heart disease in patients with HIV-1 infection? J Acquir Immune Defic Syndr. 2002;30:471-477.
15. Klein D, Hurley L, Quesenberry J, et al. Hospitalization for coronary heart disease and myocardial infarction among men with HIV-1 infection: follow-up through 12/31/03. Presented at the 11th Conference on Retroviruses and Opportunistic Infections; 2004; San Francisco.
16. Mary-Krause M, Cotte L, Simon A, et al. Increased risk of myocardial infarction with duration of protease inhibitor therapy in HIV-infected men. AIDS. 2003;17:2479-2486.
17. Currier J, Taylor A, Boyd F, et al. Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr. 2003;33:506-512.
18. Chironi G, Escaut L, Gariepy J, et al. Brief report: carotid intima-media thickness in heavily pretreated HIV-infected patients. J Acquir Immune Defic Syndr. 2003;32:490-493.
19. Depairon M, Chessex S, Sudre P, et al. Premature atherosclerosis in HIV-infected individuals-focus on protease inhibitor therapy. AIDS. 2001;15:329-334.
20. Maggi P, Serio G, Epifani G, et al. Premature lesions of the carotid vessels in HIV-1-infected patients treated with protease inhibitors. AIDS. 2000;14 (Suppl):F123-F128.
21. Meng Q, Lima J, Lai H, et al. Coronary artery calcification, atherogenic lipid changes, and increased erythrocyte volume in black injection drug users infected with human immunodeficiency virus-1 treated with protease inhibitors. Am Heart J. 2002;144:642-648.
22. Mercie P, Thiebaut R, Lavignolle V, et al. Evaluation of cardiovascular risk factors in HIV-1 infected patients using carotid intima-media thickness measurement. Ann Med. 2002;34:55-63.
23. Seminari E, Pan A, Voltini G, et al. Assessment of atherosclerosis using carotid ultrasonography in a cohort of HIV-positive patients treated with protease inhibitors. Atherosclerosis. 2002;162:433-438.
24. Hsue P, Lo J, Franklin A, et al. Increased atherosclerotic progression in patients with HIV: the role of traditional and immunologic risk factors. Presented at the 10th Conference on Retroviruses and Opportunistic Infections; 2003; Boston.
25. Dube M, Shanker S, Vanderluitgaren J, et al. Effect of indinavir (IDV) monotherapy on endothelial function in men without HIV infection. Presented at the Ninth Conference on Retroviruses and Opportunistic Infections; 2002; Seattle.
26. Monsuez J, Dufaux J, Jittecoq D, et al. Reduced reactive hyperemia in HIV-infected patients. J Acquir Immune Defic Syndr. 2000;25:434-442.
27. Nolan D, Watts G, Herrmann S, et al. Endothelial function in HIV-infected patients receiving protease inhibitor therapy: does immune competence affect cardiovascular risk? QJM. 2003;96:825-832.
28. Stein J, Klein M, Bellehumeur J, et al. Use of human immunodeficiency virus-1 protease inhibitors is associated with atherogenic lipoprotein changes and endothelial dysfunction. Circulation. 2001;104:257-262.
29. Holmberg S, Moorman A, Williamson J, et al. Protease inhibitors and cardiovascular outcomes in patients with HIV-1. Lancet. 2002;360:1747-1748.
30. Iloeja U, Yuan Y, et al. Protease inhibitor (PIs) exposure time and risk of cardiovascular disease (CVD) in human immunodeficiency virus (HIV) infected patients. Presented at the 11th Conference on Retroviruses and Opportunistic Infections; 2004; San Francisco.
31. Friis-Moller N, Sabin C, Weber R, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993-2003.
32. Stein J. Dyslipidemia in the era of HIV protease inhibitors. Prog Cardiovasc Dis. 2003;45:293-304.
33. Hui D. Effects of HIV protease inhibitor therapy on lipid metabolism. Prog Lipid Res. 2003;42:81-92.
34. Dube M, Stein J, Aberg J, et al. Guidelines for the evaluation and management of dyslipidemia in human immunodeficiency virus (HIV)-infected adults receiving antiretroviral therapy: recommendations of the HIV Medical Association of the Infectious Disease Society of America and the Adult AIDS Clinical Trials Group. Clin Infect Dis. 2003;37:613-627.
35. Executive Summary. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
36. Grundy S. Primary prevention of coronary heart disease: integrating risk assessment with intervention. Circulation. 1999;100:988-998.
37. Grundy S, Pasternak R, Greenland P, et al. Assessment of cardiovascular risk by use of multiple-risk-factor assessment equations: a statement for healthcare professionals from the American Heart Association and the American College of Cardiology. Circulation. 1999;100:1481-1492.
38. Lloyd-Jones D, Larson M, Beiser A, et al. Lifetime risk of developing coronary heart disease. Lancet. 1999;353:89-92.
39. Otvos J. Measurement of lipoprotein subclass profiles by nuclear magnetic resonance spectroscopy. In: Nader Rifai MHD, Warnick GR, eds. Handbook of Lipoprotein Testing. 2000, Washington, DC: AACC Press; 2000:609-624.
40. Otvos J. Why cholesterol measurements may be misleading about lipoprotein levels and cardiovascular disease risk-clinical implications of lipoprotein quantifications using NMR spectroscopy. Laboratoriums Medizin. 2002;26:544-550.
41. Riddler S, Smit E, Cole S, et al. Impact of HIV infection and HAART on serum lipids in men. JAMA. 2003;289:2978-2982.
42. Carr A, Miller J, Law M, et al. A syndrome of lipoatrophy, lactic acidemia and liver dysfunction associated with HIV nucleoside analogue therapy: contribution to protease-inhibitor-related lipodystrophy syndrome. AIDS. 1998;12(Suppl):F51-F58.
43. Periard D, Telenti A, Sudre P, et al. Atherogenic dyslipidemia in HIV-infected individuals treated with protease inhibitors. The Swiss HIV Cohort Study. Circulation. 1999;100:700-705.
44. Saag MS, Powderly WG, Schambelan M, et al. Switching antiretroviral drugs for treatment of metabolic complications in HIV-1 infection: summary of selected trials. Top HIV Med. 2002;10:47-51.
45. Lee I. Physical activity in women: how much is good enough? JAMA. 2003;290:1377-1379.
46. Williams P. Physical fitness and activity as separate heart disease risk factors: a meta-analysis. Med Sci Sports Exerc. 2001;33:754-761.
47. Hu F, Willett W. Optimal diets for prevention of coronary heart disease. JAMA. 2002;288:2569-2578.
48. de Lorgeril M, Salen P, Martin J, et al. Mediterranean dietary pattern in a randomized trial: prolonged survival and possible reduced cancer rate. Arch Intern Med. 1998;158:1181-1187.
49. de Lorgeril M, Salen P, Martin J, et al. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999;99:779-785.
50. Singh R, Dubnov G, Niaz M, et al. Effect of an Indo-Mediterranean diet on progression of coronary artery disease in high risk patients (Indo-Mediterranean Diet Heart Study): a randomised single-blind trial. Lancet. 2002;360:1455-1461.
51. Knowler W, Barrett-Connor E, Fowler S, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
52. Tuomilehto J, Lindstrom J, Eriksson J, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343-1350.
53. Aberg J, Zackin R, Evans S, et al. A prospective, multicenter, randomized trial comparing the efficacy and safety of fenofibrate versus pravastatin in HIV-infected subjects with lipid abnormalities: ACTG 5087. Presented at the XIV International AIDS Conference; 2002; Barcelona.
54. Ballantyne C, Davidson M. Possible differences between fibrates in pharmacokinetic interactions with statins. Arch Intern Med. 2003;163:2394-2395.
55. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7-23.
56. Sever P, Dahlof B, Poulter N, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149-1158.
57. Cannon C, Braunwald E, McCabe C, et al. Comparison of intensive and moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495-1504.
58. Nissen S, Tuzcu E, Schoenhagen P, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291:1071-1080.
59. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. Circulation. 2004;110:227-239.
|
|
| |
| |
|
|
|