| |
Assessment of the safety and efficacy of poly-L-lactic acid for the treatment of HIV-associated facial lipoatrophy
|
| |
| |
REPORT
Journal of the American Academy of Dermatology
February 2005, part 1, Volume 52, Number 2
Cheryl M. Burgess, MD *
Rafaela M. Quiroga, MD
From the Center for Dermatology and Dermatologic Surgery
Funding sources: None.Conflicts of interest: None identified.
Accepted August 30, 2004
Sections
* Abstract
* Methods
* Results
* Discussion
* Acknowledgements
* References
ABSTRACT
Background Lipodystrophy syndrome is uniquely associated with the use of highly active antiretroviral therapy (HAART) containing protease inhibitors or nucleoside reverse transcriptase inhibitors. Between 15% and 80% of patients on HAART develop facial lipoatrophy within 10 months of initiating therapy. At present, no ideal treatment strategies have emerged in spite of the psychosocial stress, resulting in depression and isolation in many HIV-infected patients. Most soft tissue fillers seem to be well tolerated; however, various reactions such as allergic reactions, infection, and inflammatory and allergic granulomatous nodules are possible. Poly-L-lactic acid (PLA; New-Fill, Biotech Industry SA, Luxembourg) is currently being used in Europe and is approved by the Food and Drug Administration (FDA) in the United States for soft tissue augmentation in HIV-associated facial lipoatrophy.
Objective To determine the safety and efficacy of PLA for dermal enhancement of facial lipoatrophy in immuncompromised HIV-infected patients with prior use of HAART.
Methods Sixty-one immunocompromised, HIV-infected male patients (52 whites, 7 African Americans, 1 Latino, and 1 Asian) underwent multiple treatment sessions with PLA over a 5-month period for facial lipoatrophy. The severity of facial lipoatrophy was assessed and photographs were taken at baseline and before each treatment session. Periodic monitoring for adverse reactions and degree of improvement were assessed by the patient, the treating physician, and a non-treating physician.
Results At the 6-month follow-up, all 61 immunocompromised HIV patients had a successful outcome, defined as smoothing of the skin with less concavities or depressions, and improved overall appearance in an average of 3 treatment sessions. Although all patients were very pleased with their results, two patients developed persistent asymptomatic palpable intradermal papules in the infraorbital region as a result of the site of placement and concentration of PLA. On long-term follow-up (18 months), 48 of 61 (79%) required an average of 3 visits to achieve the desired enhancement and 13 of 61 (21%) patients requested additional treatment sessions beyond the initial 3 sessions. Although the patient and the physicians rated the level of improvement as "Excellent," the desire for further dermal enhancement was purely subjective. In general, the procedures were well tolerated without the clinical development of adverse reactions.
Conclusion The use of PLA to treat facial lipoatrophy resulted in significant and prolonged improvement in HIV-infected patients. The effect was long lasting, for up to 2 years in some patients, depending on when treatment was initiated. There were no reported cases of infection, allergies, or serious adverse reactions, and the treatment was well tolerated.
INTRODUCTION
Although lipodystrophy syndrome has been proven difficult to define and the etiology is unclear, studies suggest a strong link between HIV-associated lipodystrophy syndrome and treatment with protease inhibitors (PIs), and a number of nucleoside reverse transcriptase inhibitors (NRTIs) may also be involved.1,2 In addition to clinical findings such as lipoatrophy and fat redistribution, many agree that alterations in the lipid profiles and glucose intolerance are included within the definition of lipodystrophy syndrome.2 The estimates of prevalence of lipodystrophy syndrome vary in the range of 15% to over 80%.3 The highest estimate comes from Carr and Cooper,2 who reported the development of lipodystrophy syndrome in 83% of patients within 8 to 10 months of initiating PI therapy.
Growing recognition of the psychological stress caused by facial lipoatrophy has driven investigation of several treatment strategies. Current treatments under examination include autologous fat transfer and temporary, semi-permanent, and permanent facial fillers such as bovine4 and human collagen, hyaluronic acid,3,4 poly-L-lactic acid (PLA),5,6 polymethylmethacrylate fillers,3 and injections of silicone7,8 in addition to cheek implants and other synthetic materials such as Gore-Tex strips.2,8,9
At present, no ideal treatment strategy has emerged. Treatments have either a short or permanent duration of correction, while others may carry a potential risk of allergic response.4,8 Lemperle et al10 hypothesize that all injectable filler substances can cause normal foreign body-type reaction that may develop into a foreign body granuloma in selected patients.
METHODS
Patient selection
Over the course of 24 months (October 2001 to September 2003), primary care physicians referred 61 immunocompromised, HIV-positive men (52 whites, 7 African Americans, 1 Latino, and 1 Asian) to the Center for Dermatology and Dermatologic Surgery in Washington, DC for evaluation and treatment of facial lipoatrophy. (Note: One patient was referred for lipoatrophy around the knee.) Demographic characteristics, HIV history, highly active antiretroviral therapy (HAART) use, and duration of lipoatrophy are listed in Table I.
Table 1. Demographic characteristics
Age: 45.5 (35-74
Years since HIV diagnosis: 7.18 (2-17)
Years with lipodystrophy syndrome: 3.72 (1-15)
Patients with prior treatments for facial lipoatrophy: 11
Sixty-one patients had a current or past history of being treated for at least 1 year with HAART/PI. All patients were deeply concerned about their physical appearance. Eleven patients had been previously treated for the correction of their facial wasting. Previous treatments included autologous fat transfer, cheek implants, and various dermal enhancements, such as autologous and bovine collagen, dermoplasty,11 and silicone oil injections. All patients were screened for anticoagulant medications or agents. One patient's history was significant for hemophilia type A deficiency of plasma factor VIII. No patients were excluded from receiving PLA dermal enhancement.
Materials
PLA is a biodegradable bioabsorbable aliphatic polyester12 produced by carbohydrate fermentation of corn dextrose. The lyophilizate is composed of polylactic acid microspheres suspended in an apyrogenic mannitol and sodium cellulose preparation. PLA has been utilized for years in resorbable surgical material, such as sutures, plates and screws, and membranes for guided-tissue regeneration in periodontal surgery.13
For this study, PLA was obtained by individual patients using the personal use importation (PUI) process, regulated by the Federal Drug Administration (FDA). The PUI requires that the foreign drug or device be distributed non-commercially in volumes not considered excessive (ie, a 3-month supply or less). The FDA also stipulates that the intended use of the drug or device be appropriately identified and that a patient affirms in writing that the treatment is for personal use, including the name and address of the licensed physician in the United States responsible for his or her treatment with the drug or device.14
Study design
To document baseline appearance of each patient, a preoperative series of digital and 35 mm photographs were taken of the face consisting of left and right lateral, left and right oblique, and frontal views.
Treatment sessions for the areas of facial lipoatrophy were performed every 3 to 6 weeks over a 5-month period. Subjective evaluation before each treatment session assessed the patient's level of improvement that most represented their perception of their current state of improvement in appearance from the treatment evaluation scale (Table II). Additionally, the treating physician and a non-treating physician assessed the skin for improvement and adverse reactions every 6 months by comparing the patient's current appearance to the baseline photographs.
Table II. Treatment evaluation scale
1 Excellent (90-100% improvement)
2 Good (50-89% improvement)
3 Fair (less than 50% improvement)
4 No change (no improvement)
Procedure
A topical anesthetic cream (EMLA containing 2.5% lidocaine [Astra AB, Sdertlje, Sweden]; Ela-Max 5% lidocaine cream [Max-Ferndale Laboratories, Inc, Ferndale, Mich]; or Betacaine LA, containing 15% lidocaine and 5% prilocaine [Custom Scripts Pharmacy, Tampa, Fla]) was applied to the area to be enhanced and allowed to remain for at least 1 hour. After the anesthetic cream was removed, the skin was cleansed with two antibacterial preparations. First, a 10% povidone-iodine solution was applied and allowed to remain on the skin for several minutes. The povidone-iodine was then removed with isopropyl alcohol. Patients who received topical Ela-Max, EMLA, or lidocaine cream 5% received also infraorbital nerve blocks and ring blocks with 1% lidocaine HCl (Xylocaine; Astra USA, Westborough, Mass) to the affected areas of the face; most patients who received Betacaine did not require nerve blocks. The patient with a history of hemophilia A required factor VIII infusions at least 1 hour before dermal injections.
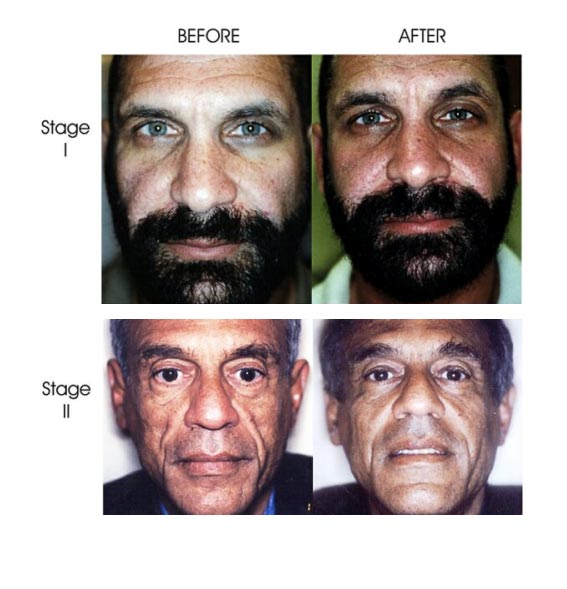
The classification of facial lipoatrophy in each patient was expressed according to a scale describing the location of fat wasting and type of desired enhancement (Table III). The approximate number of PLA vials used was determined by lipoatrophy staging: stage I: 1-2 vials; stage II: 2 vials; stage III: 3-4 vials; stage IV: 4 vials.
Table III. Staging of facial lipoatrophy by area of involvement
Description
Stage I Minimal fat wasting of the cheeks only and minimal enhancement of the nasolabial folds
Stage II Moderate fat wasting of the cheeks with enhancement of the nasolabial fold region, and appearance of "nasolabial bands"
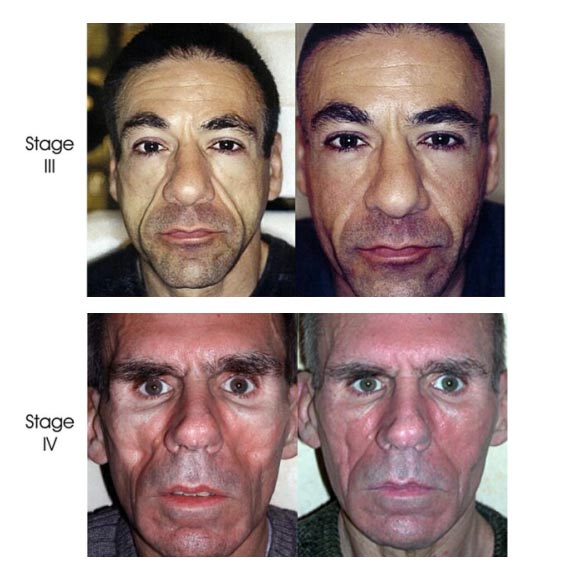
Stage III Moderate fat wasting of the cheeks and temporal regions in addition to prominent nasolabial folds region "nasolabial bands"
Stage IV Severe fat wasting and hallowing of the cheeks, temporal, and periocular regions in addition to visible facial bony prominences
The PLA vial of 150 mg was reconstituted to form a hydrogel with 4 cc to 6 cc of bacteriostatic water at least 1 hour before dermal injections. PLA was injected into the junction of the subcutaneous-deep dermal plane using a 3 cc syringe with a 25-gauge 11Ú2 inch needle. A "fanning" injection technique was used to create a deep dermal lattice. Skin molding and sculpturing was accomplished by the use of the palm and thumb or fingertip thimbles over the skin in a "rolling pin" maneuver.
Postoperative care consisted of routine facial cleansing with hydrogen peroxide and application of moisturizer with sun protection.
Evaluation
PLA injections produce an immediate enhancement at the time of the treatment session and a delayed enhancement that becomes more apparent after 2 months. Skin evaluations were made by the treating physician, a non-treating physician, and the patient before each treatment session, 2 months after the final treatment, and every 6 months thereafter, by comparing the patient's current appearance to the pre-treatment photographs. Based on a visual and subjective analysis, a 4-point improvement scale was used for evaluation (Table II). Patients requesting additional treatment sessions (4 or more) were also assessed with the 4-point improvement scale before the dermal enhancement procedure.
RESULTS
Demographic characteristics, HIV history, HAART use, and duration of lipoatrophy are listed in Table I. The distribution of severity among patients was stage I (n=9); stage II (n=15); stage III (n=30); and stage IV (n=7). After the initial treatment, 100% of the patients noted at least a slight improvement of facial concavities (grade 3, or "Fair"). In all cases, the patient rated his appearance higher than did the physicians. This was true even when the physicians described "No Change." Two patients clinically developed palpable and occasionally visible intradermal papules in the infraorbital region as a result of the placement of the 4 cc reconstituted PLA concentration, and 5 patients developed the appearance of minor bruising within 3 days of the procedure, lasting no more than 7 days. There were no incidents of swelling, edema, allergic reactions, infection, erythema, or clinical evidence of adverse reactions.
Based on a visual, subjective analysis using a 4-point scale (Table II), the patient and both physicians assessed the effectiveness of treatment in achieving a completely by healthy appearance.
At the 6-month follow-up evaluation, 100% of patients and both physicians agreed to "Excellent" responses, and a complete achievement of a healthy appearance. Seventy-nine percent of patients achieved this result in less than 4 sessions and 21% of patients requested 4 or more additional treatment sessions.
Following 3 treatment sessions, significant improvement and dermal thickening were retained in 37 patients for a duration of 6 months; in 10 patients for a duration of 1 year; in 9 patients for a duration of 18 months; and in 5 patients for a duration of 2 years or more. Thirteen patients requested touch up sessions at an average of 1 year; however, on evaluation by the physicians, there was no change from the 90% to 100% dermal enhancement improvement grade.
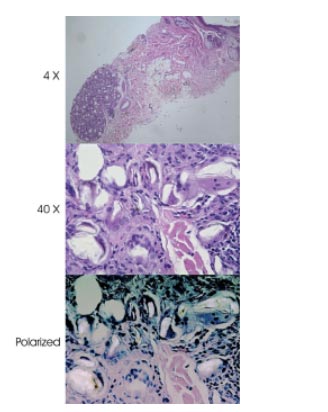
Histologic evaluations were performed on two patients at 0 to 3 months, 3 to 6 months, and at 18 months. Specimens were also obtained from 2 patients with persistent asymptomatic intradermal papules in the infraorbital region. The specimens were used to verify proper location and reaction of the filler material (Figs 1 and 2). The histologic assessment with polarized light microscopy reported an intradermal mass of birefringent material consisting of deep dermal granulomas composed of histiocytes, many multinucleated giant cells, and foreign material within some of the giant cells. Because of the presence of many multinucleated giant cells around the foreign body, the histologic description was that of a classic foreign-body granuloma.
Fig 1. Facial lipoatrophy. Stage I: minimal fat wasting (of the cheeks only) and minimal enhancement of the nasolabial folds. Stage II: moderate fat wasting of the cheeks with enhancement of the nasolabial fold region and appearance of "nasolabial bands." Stage III: moderate fat wasting of the cheeks and temporal regions in addition to prominent nasolabial folds region (nasolabial bands). Stage IV: severe fat wasting and hallowing of the cheeks, temporal, and periocular regions, resulting in visible facial bony prominences.
|
|
| |
 |
 |
| |
| |
Fig 2. Intradermal mass consisting of deep dermal granulomas composed of histiocytes, many multinucleated grant cells, and foreign material within some giant cells. (Hematoxylin-eosin stain; original magnifications: top, ~4; middle, ~40; bottom, ~40 polarized.)
|
|
 |
| |
| |
| |
All patients who received PLA dermal enhancement since October 2001 have been followed every 6 months for possible adverse events and hypersensitivity reactions. At the time of writing, there have been no reported allergic reactions, infections, or adverse events.
AUTHOR DISCUSSION
HIV-associated lipodystrophy syndrome consists of fat loss from the limbs, buttocks, and face; the redistribution and fat accumulation in the abdomen and dorso-cervical fat pad (the so-called "buffalo hump"); occasional breast hypertrophy; alteration in lipid profiles; and glucose intolerance.1,2 The most visible component of fat redistributionŃthe component that causes the patient the most distressŃis facial lipoatrophy.16
Poor body image, low self-esteem, and depression were frequently described. Perhaps most significantly, some patients stopped taking their antiretroviral regimen in order to avoid the adverse psychosocial effects of fat wasting.16
Facial lipoatrophy begins in the region of the cheeks, progresses outward to the temples, and finally affects the periocular region. As fat loss progresses, the facial skin comes to rest directly on the underlying muscles, resulting in a gaunt, anorexic appearance. To classify the severity of facial lipoatrophy, James et al2 proposed a 4-point scale in which grade I to grade IV reflects least to greatest severity (Table IV). Additionally, we have proposed a 4-point staging classification to reflect the clinical progression of the areas of involvement in HIV-associated facial lipoatrophy (Table III).
Table IV. Facial lipoatrophy severity scale2
Description
Grade I Mild and localized facial lipoatrophy.
Grade II Deeper and longer atrophy, with the facial muscles beginning to show through.
Grade III Atrophic area is even deeper and wider, with the muscles clearly showing.
Grade IV Lipoatrophy covers a wide area, extending up toward the eye sockets, and the facial skin lies directly on the muscles.
Many treatments have been explored. Each of these treatments has its advantages, but none is perfect. The ideal dermal filler would be completely non-allergenic; have a long duration of effect, but not so permanent that errors in treatment could not be corrected; and produce a smooth, natural-appearing result.
Although autologous fat transfer carries no risk of immune response, the transferred fat is still subject to the same wasting effects as the original fat. Approximately 50% of injected fat will disappear after 6 months.17 Finding enough harvestable fat can also be problematic in the HIV population. The Fat Redistribution and Metabolic Change in HIV Infection Study (FRAM) examined HIV-infected persons in comparison with healthy controls. The FRAM study found that persons with HIV actually lost more central fat than the control population. It seems that the apparent central fat accumulation may only appear to be an accumulation in comparison to the larger fat loss elsewhere on the body.18,19
With the increasing demand for soft tissue augmentation with soft tissue filler substances, numerous agents been investigated for safety and efficacy. It has been reported that all injectable filler substances have been known to cause foreign body-type reactions in select patients.10,13 The various resorbable, biodegradable, and permanent fillers found to elicit a foreign body reaction have been: collagen (Zyplast; McGhan Medical Corp, Fremont, Calif), hyaluronic acid (Restylane; Q-Med AB, Uppsala, Sweden),20 PMMA microspheres (Artecoll; Canderm Pharma, St. Laurent, Quebec, Canada), silicone oil (PMS 350; Dow-Corning, Midland, Mich), dextran microspheres (Reviderm Intra; Canderm Pharma), polymethylacrylate particles (Dermalive, Quebec, Canada), and polylactic acid (New-Fill).10,13,21
Host immune mechanisms account for the reactions to various filler materials10 and the patient's ability to react to these fillers may be altered or suppressed in the HIV/AIDS patient population.
When PLA is injected into the deep dermis or subcutaneous-deep dermal plane, it causes an immediate tissue expansion and physical improvement to the appearance of the skin which is purely mechanical. Once the carrier solution is reabsorbed, a slow process of biodegradation of the microspheres occurs. The process of hydratation, loss of cohesion and molecular weight, solubilization and phagocytosis of PLA by the host's macrophages13 slowly degrades the PLA into lactic acid microspheres and eliminates CO2 (via lactate/pyruvate route) by way of respiratory excretion, thus leaving behind the crystals to stimulate collagen and a granulomatous reaction.12,22,23,24 This inflammatory reaction elicits resorption and the formation of fibrous connective tissue about the foreign body resulting in dermal fibroplasia25,26 which is aimed at producing the cosmetic result. This process is estimated to go on for an average of 10 to 12 months, with extremes of 7 months to 2 years.13
The initial effect fades over the first week as the water is absorbed, causing a temporary return to at or near the pre-treatment appearance.13 It takes several months for treatment results to stabilize and for the full magnitude of the facial augmentation to become apparent.
A European study followed 26 men who were given 3 mL injection of PLA for 2 weeks. Subsequent physical examinations and echography showed dermal thickness increasing by 4.1 mm at 12 weeks and by 5.1 mm at 24 weeks. The majority of patients undergoing the procedure claimed resolution of their facial abnormalities and no adverse reactions were reported.5
Although studies in HIV-related facial lipoatrophy are ongoing, the use of PLA for dermal enhancement in HIV patients is too new for conclusive data to exist on the longevity of its effect and its potential immune response. Reports regarding use in eliminating wrinkles suggest that such results remain for 12 to 18 months; at that point only one booster treatment is needed to maintain the cosmetic effect. Polo and colleagues27 confirmed previously reported good results with polylactic acid. Additionally, the results of the VEGA open label study involving 50 HIV infected patients reported that the benefits of PLA were clearly demonstrated with an evident aesthetic and quality of life improvement.6
We found the recommended reconstitution dilution of 3 cc of bacteriostatic water to be difficult to inject through the 26 gauge needles supplied in the kit and that this concentration resulted in persistent asymptomatic intradermal papules in our patients.28 Additionally, we determined that reconstitution at any dilution caused the PLA to clump in the syringe and was somewhat difficult to inject if sufficient time was not allotted for complete covalent bonding of the lactic acid monomers to form PLA. Palpable and occasionally visible papules or blanching of the skin occurred with superficial dermal injections. Correct placement in the subcutaneous-deep dermal plane and the concentration were equally important when injecting near the orbital region. Superficial placement of the hydrogel, the immune status of the individual, and/or the location of enhancement could possibly account for the formation of clinically apparent inflammatory nodules as reported by Saylan et al.21 PLA should only be injected by cosmetic surgeons or dermatologists who have been specifically trained in its use and dermal placement. We also recommend adherence to standard occupational safety precautions as defined by the Centers for Disease Control and Prevention (CDC) and the Occupational Safety and Health Administration (OSHA) enforcement for all personnel who may come in contact with blood or infectious material.
Among the newest injectable agents for HIV-associated facial lipoatrophy is PLA. Injection of PLA into the subcutaneous-deep dermal plane results in dermal fibroplasia and cutaneous thickening over a period of months without the incidence of serious adverse events in this patient population. All patients treated agreed with their doctors that normal, healthy facial appearance had been restored after an average of 3 treatments, regardless of severity; and it was evident that the quality of life assessment had improved since the initial consultation.
Because of the physical properties, a foreign body response followed by the slow resorption of the microspheres, and subsequent dermal fibroplasia, the volume and lasting effect of PLA in the treatment of cutaneous depressions are maintained.8,25 We found that significant improvement and dermal enhancement was maintained for an average of 18 months. Our experience has shown that the use of PLA in the treatment of HIV-associated facial lipoatrophy was well tolerated by all patients and there were no reported cases of persistent swelling, edema, infection, or any clinical evidence of adverse reaction.
References
1. Dieterich DT. Long-term complications of nucleoside reverse transcriptase inhibitor therapy AIDS Read 2003;13:176-184. 87
2. James J, Carruthers A, Carruthers J. HIV-associated facial lipoatrophy Dermatol Surg 2002;28:979-986.
3. Lemperle G, Romano JJ, Busso M. Soft tissue augmentation with artecoll: 10-year history, indications, techniques, and complications Dermatol Surg 2003;29:573-587.
4. Narins RS, Brandt F, Leyden J, Lorenc P, Rubin M, Smith S. A randomized, double-blind, multicenter comparison of the efficacy and tolerability of Restylane versus Zyplast for the correction of nasolabial folds Dermatol Surg 2003;29:588-595.
5. Amard P, Saint-Marc T, Katz P. The effects of polylactic acid as therapy for lipodystrophy of the face Antivir Ther 2000;5:76. Abstract P94
6. Valantin MA, Aubron-Olivier C, Ghosn J, Laglenne E, Pauchard M, Schoen H. Polylactic acid implants (New Fill) to correct facial lipoatrophy in HIV-infected patients: results of the open-label study VEGA AIDS 2003;17:2471-2477.
7. Jones DH. Injectable silicone for facial lipoatrophy Cosmet Dermatol 2002;15:13-15.
8. Orentreich D. Liquid injectable silicone: techniques for soft tissue augmentation Clin Plast Surg 2000;27:595-612.
9. Conrad K, Reifen E. Gore-Tex implant as tissue filler in cheek-lip groove rejuvenation J Otolaryngol 1992;21:218-222.
10. Lemperle G, Morhenn V, Charrier U. Human histology and persistence of various injectable filler substances for soft tissue augmentation Aesth Plast Surg 2003;27:254-266.
11. Hwang K, Han JY, Kim DJ. Dermofat graft in deep nasolabial fold and facial rhytidectomy. In: Aesthetic Plast Surg 2003;27:254-7.
12. Vert M, Li SM, Garreaw H. Attempts to map the structure and degradation characteristics of aliphatic polyesters derived from lactic and glycolic acids J Biomater Sci Pulm Ed 1994;6:639-649.
13. Lombardi T, Samson J, Plantier F, Husson C, Kuffer R. Orofacial granulomas after injection of cosmetic fillers. Histopathological and clinical study of 11 cases J Oral Pathol Med 2004;33:115-120.
14. Administration USFaD. Policy on importing unapproved AIDS drugs. Vol. 2004, 1988.
15. Lever WF, Schaumburg-Lever G. Non-infectious granulomas In Histopathology of the Skin, Histopathology of the Skin8th ed.1997Lippincott-RavenPhiladelphia, Pa, eds Philadelphia, Pa: Lippincott-Raven; 1997. p. 317-340.
16. Collins E, Wagner C, Walmsley S. Psychosocial impact of the lipodystrophy syndrome in HIV infection AIDS Read 2000;10:546-550.
17. Horl HW, Feller AM, Biemer E. Techique for liposuction fat reimplantation and long-term volume evaluation by magnetic resonance imaging Ann Plast Surg 1991;26:248-258.
18. Moyle G. Lipodsytrophy: lack of agreement on definition and etiology presents a challenge to research and therapy AIDS Read 2002;12:440-442.
19. Hartmann M, Petzoldt D. Lipodystrophy syndrome in HIV infection Hautarzt 2000;51:159-163.
20. Lupton JR, Alster TS. Cutaneous hypersensitivity reaction to injectable hyaluronic acid gel Dermatol Surg 2000;26:135-137.
21. Ziya Saylan MD. Facial fillers and their complications Aesth Surg 2003;23:221-224.
22. Gruber PR, Hall ES, Kolstad JH, Iwen ML, Benson RD, Borchardt RL, inventors. Continuous porcess for manufacture of lactide polymers with controlled optical purity. US patent 5142023. 1992
23. Micard V, Guilbert S. Int J Biol Macromolecules 2000;27:216-229.
24. Hartman MH. Biopolymers from renewable resources In , eds D.L. Kaplan. Berlin: Springer-Verlag; 1998. p. 367.
25. Cutright DE, Hunsuck EE. Tissue reaction to biodegradable polylactic acid suture Oral Surg 1971;31:134-139.
26. Kulkarni RK, Pani KC, Neuman C, Leonard F. Polylactic acid for surgical implants Arch Surg 1966;93:839-843.
27. Polo R, Ortiz M, Babe J, Martinez S, Madrigal P, Gonzalez-Munoz M. The effects of polylactic acid in the therapy for the lipoatrophy of the face. Program and abstracts of the 3rd International Workshop on Adverse Drug Reactions and Lipodystrophy in HIV; October 23-26, 2001; Athens, Greece Antiviral Ther 2001;6:68. Abstract 101
28. New-Fill [package insert]. Luxembourg: Biotech Industry SA; 2004.
|
|
| |
| |
|
|
|