| |
TMC114/r in 3-Class Experienced Patients: 24-week interim analysis
|
| |
| |
Reported By Jules Levin
Access to this new PI for patients with extensive experience to PIs and few treatment options is available now and in the Fall through studies of this drug for treatment-experienced individuals, including a study for highly treatment-experienced patients. The company, Tibotec, announced an Expanded Access Program is expected soon. The company also announced that in early 2006 they expect to file with the FDA an application for accelerated approval based on early 24-week data from the phase IIb study. Such an early approval based on data this preliminary is unusual as results from large phase III studies are usually the basis for approval. If you are interested in access you can speak to your doctor. This report contains the data results from the phase IIb study and additional information on studies & access. Company contacts for additional information:
Barbara Sabatino 609-730-7549 bsabatin@tibus.jnj.com
Gino Van Dooren 32-15-292-484 gvdooren@tibbe.jnj.com
Here is link to studies & sites:
TMC114: two studies for patients with treatment-experience
class="body">http://www.natap.org/2005/HIV/062205_03.htm
"Antiretroviral Therapy: New Agents"
At the 4pm oral session Friday on the last day of the 12th CROI held in early 2005, encouraging and promising new information was presented for several new antiretroviral drugs in various stages of development. I think everyone walked out of this session smiling because the data and promise for new drug development looks promising. The most important presentation was the 24-week interim analysis of the efficacy of TMC-114/r, a new protease inhibitor for patients with extensive PI & ART resistance. The second exciting advance was the first time data in patients was presented publicly for Merck's integrase inhibitor currently in phase II study in patients . Third, Panacos Pharmaceuticals presented promising results from giving patients single-dose therapy of their new drug PA-457, a maturation inhibitor. And Tibotec also presented first-time new data for TMC278, a new NNRTI that is active against NNRTI resistance.
"Efficacy of TMC114/r in 3-Class-Experienced Patients with Limitred Treatment Options: 24 week planned interim analysis of two 96-week multi-national studies"
SUMMARY:
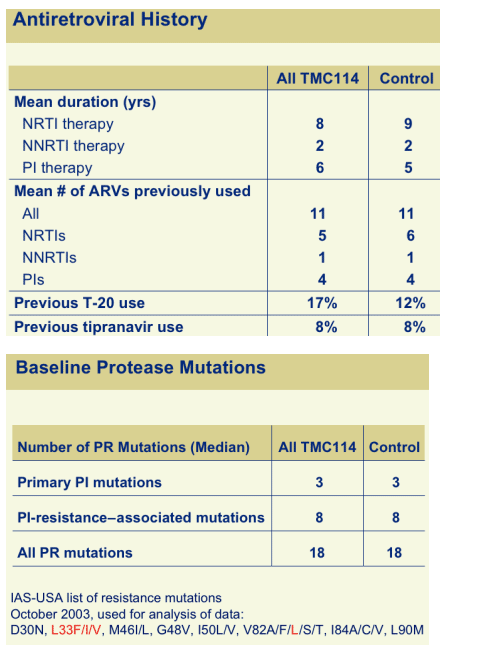
--study patients had extensive treatment-experience; used average of 11 prior ARVs, 4 PIs, 1 NNRTI, 5 NRTIs. 15% previously used T20. Patients had extensive resistance to PIs: LPV/r 80-fold; ATV 38-fold; SQV 29-fold; APV 19-fold; TMC114 4-fold; patients had 18 protease mutations.
--Efficacy of TMC114 was greatest with 600 mg b.i.d. and significantly better than control
--Sustained mean VL reduction 1.85 vs. 0.27 log for control group
--Patients with >1 log reduction 72% vs. 16% for control group
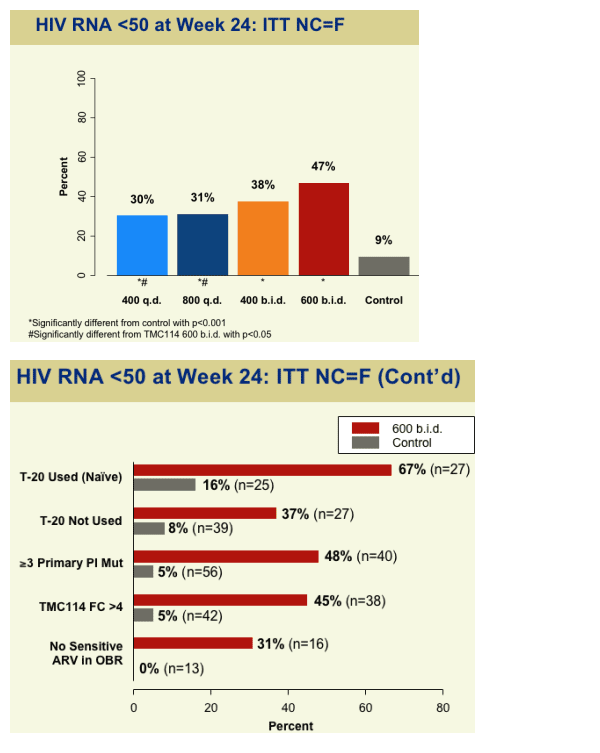
--Patients with <50 copies/mL 47% vs. 9% for control group
--Mean CD4 cells/mm3 increase 75 vs. 15 for control group
--patients who used T20 (naive) : 67% in 600mg bid group had <50 copies/ml (ITT NC=F); patients with 3 or more primary PI mutations: 48% had <50 copies/ml; patients with no sensitive ARV in OBR: 31% had <50 copies/ml
--TMC114 safety and tolerability was comparable to available boosted PI regimens and not dose dependent
--TMC114/RTV 600/100 mg b.i.d. shows promise for 3-class–experienced patients with limited treatment options and has been selected for Phase 3 studies
The presentation included results from two dose-finding studies (TMC114-C213/C202). Patients were partially blinded, to dose). Patients were equally randomized to 1 of 4 TMC114 doses (given with lo-dose RTV) or investigator selected PI regimen in combination with optimized background regimen:
--400/100r and 800/100r mg once daily (qd)
--400/100r and 600/100r mg twice daily (bid)
--Control: investigator selected PI regimen
Total treatment duration is 96 weeks.
The study is multinational & multicenter in 14 countries at 96 participating sites.
The primary objective/end point is to evaluate the relationship between dose of TMC114 & antiviral response (change in HIV RNA) at 24 weeks.
RANDOMIZATION STRATIFICATION FACTORS
--T20 use (yes, no)
--Baseline viral load (<20,000, >20,000)
--number of primary PI mutations (1, 2, ≥3)
VIROLOGIC FAILURE
--liss than 0.5 log reduction in HIV RNA >8 weeks
--less than 1.0 log reduction >12 weeks
--2 consecutive measurements of plasma HIV RNA greater than 0.5 log above nadir
PLANNED INTERIM ITT: subjects who changed/discontinued prior to week 24 had their change in HIV RNA & CD4 imputed to 0.
INCLUSION CRITERIA
--at least 3-class experienced (PI, NRTI, NNRTI)
--currently receiving a PI regimen
--at least 1 primary PI mutation (ant combination, IAS-USA list March 2003)
--plasma HIV RNA >1000 copies/ml
--no CD4 count restrictions
--coinfection with hepatitis C or B allowed in TMC114-C213.
Patients were mostly resistant to available PIs except to TMC114.
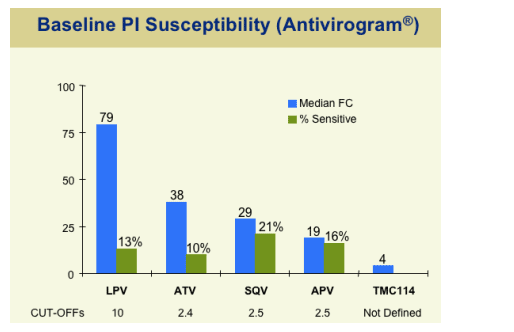
Tipranavir baseline Phenotypic Susceptibility Profile presented by the FDA at public hearing:
Study patients had on average 80-fold resistance to LPV/r (Kaletra), 38-fold to atazanavir, 29-fold to SQV, 19-fold to amprenavir, and were 4-fold resistant to TMC114, to which no clinical cutoff has been developed.
BASELINE PROTEASE MUTATIONS

IAS-USA list of resistance mutations
October 2003, used for analysis of data:
D30N, L33F/I/V, M46I/L, G48V, I50L/V, V82A/F/L/S/T, I84A/C/V, L90M
USE of T20 in OBR & I USED in CONTROL GROUP
48% of patients receiving TMC114 used T20: 35% were T20 naïve & 15% were T20 experienced. 41% in the control group used T20.
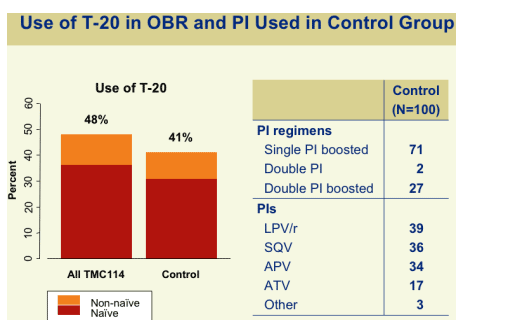
CONTROL Group
N=100
PI Regimens
--single PI boosted 71
--double PI 2
--double PI boosted 27
PIs
LPV/r 39
SQV 36
APV 34
ATV 17
Other 3
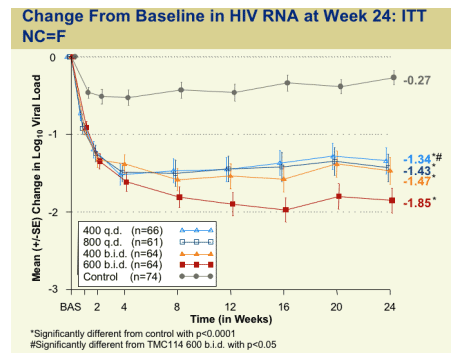
Change From Baseline in HIV RNA at Week 24: ITT NC=F
Week 24:
600 bid (n=64): -1.85 log
400 bid (n=64): -1.47 log
800 qd (n=61): -1.43 log
400 qd (n=66): -1.34 log
control: -0.27 log
CD4 Count: mean change from baseline (ITT M=F:
+75 cells for 600 bid
+50-55 for the other 3 TMC dose groups vs +15 for controls.
≥1 Log10 Reduction at Week 24: ITT NC=F
600 bid: 72%
400 bid: 58%
800 qd: 56%
400 qd: 55%
control: 16%
HIV RNA <50 at Week 24: ITT NC=F
600 bid: 47%
400 bid: 38%
800 qd: 31%
400 qd: 30%
control: 9%
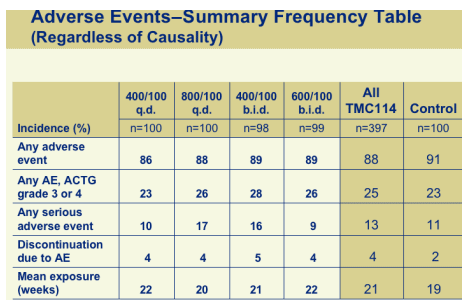
ADVERSE EVENTS
There was no difference between the 600 bid arm, the All TMC114 & Control groups in numbers of any adverse events, any AE (ACTG grade 3 or 4), any serious event, discontinuation due to AE or mean exposure (weeks). ACTG grade 3 or 4: 26 (600/100), 25 (all TMC114), 23 Control. Any serious AE: 9 in 600/100, 13 all TMC114, 11 Controls. Disct due to AE: 4 (600/100), 4 all TMC114, 2 Controls.
The most common adverse events were headache (15%), diarrhea (15%), nausea (17%), insomnia (3%), fatigue (7%) in 600/100. In each case Controls had higher percentages.
LAB ABNORMALITIES
Grade 3 or 4
--GGT: 28% TMC114, 1% Controls
--AST: 1.8% TMC114, Controls 3%
--ALT: 1% TMC114, Control 1%
--no liver related SAE was reported during treatment on TMC 114
--safety & lab profiles of coinfected HBV & HCV patients were similar to overall population.
LIPIDS
Grade 3 or 4:
--triglycerides: TMC114 6.6%, Control 6%
--total cholesterol: 1.5% TMC114, Control 1%
|
|
| |
| |
|
|
|