| |
Effect of Antioxidants on Glucose Metabolism and Plasma Lipids in HIV-Infected Subjects With Lipoatrophy
|
| |
| |
JAIDS Journal of Acquired Immune Deficiency Syndromes: Volume 33(5) 15 August 2003 pp 605-607
McComsey, Grace*; Southwell, Heather; Gripshover, Barbara; Salata, Robert; Valdez, Hernan
*Rainbow Babies and Children's Hospital, and Center for AIDS Research, Case Western Reserve University and University Hospitals of Cleveland, Ohio
Supported by a grant from the Campbell Foundation.
Summary:
Ten HIV-infected nucleoside reverse transcriptase inhibitor-treated subjects with lipoatrophy or sustained hyperlactatemia were given antioxidants: vitamins C, E, and N-acetyl cysteine. After 24 weeks, anthropometrics did not change significantly, except for a modest decrease in the waist-to-hip ratio. Fasting low-density lipoprotein cholesterol trended toward lower values. Fasting glucose significantly increased along with a significant increase in homeostatic model assessment values, reflecting an increase in insulin resistance. Controlled trials are required to evaluate directly the effects of these agents on lipid and glucose metabolism.
.....The antioxidants used in this pilot trial were well tolerated, except for an increase in fasting glucose and in HOMA-IR, a widely used index of insulin resistance, which accounts for both fasting insulin and glucose levels and is known to correlate well with clamp techniques. These findings are of concern and suggest increasing insulin resistance. Alternatively, this could represent the natural history of insulin resistance in lipoatrophy. Favorable trends toward lower low-density lipoprotein cholesterol and waist-to-hip ratios are encouraging.....
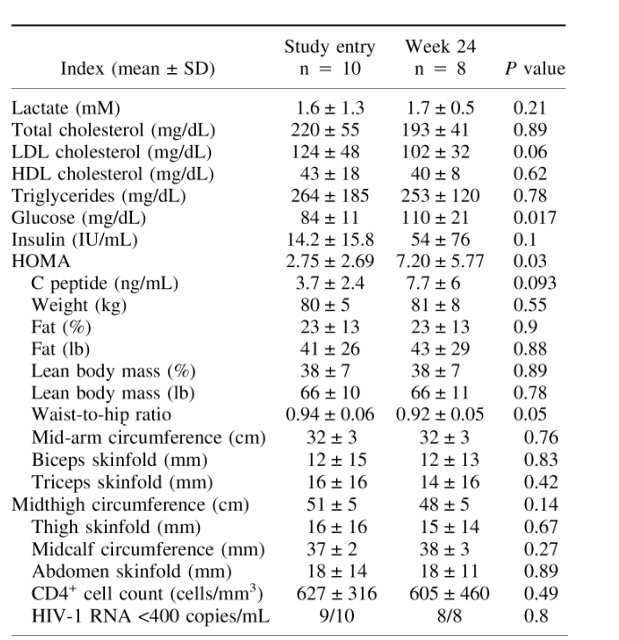
TABLE 1. Baseline characteristics and changes after 24 weeks of N -acetyl cysteine and vitamins E and CLDL, low-density lipoprotein; HDL, high-density lipoprotein; HOMA, homeostatic model assessment.
Lipoatrophy and hyperlactatemia are well-recognized complications of antiretroviral therapy that have been linked to nucleoside reverse transcriptase inhibitor (NRTI)-induced mitochondrial toxicity. 1-4 The exact mechanism of this mitochondrial dysfunction remains unclear, but several studies suggested a link to increased oxidative stress. Zidovudine has been shown to increase 8-oxo-7,8-dihydro 2-deoxyguanosine (8-oxo-dG), a marker of oxidative damage to DNA. 5 Antioxidants (vitamins C and E) resulted in significant decrease in 8-oxo-dG and prevented morphologic changes of muscle mitochondria in zidovudine-treated mice. 6 Similar changes in oxidative markers were seen with stavudine therapy, and these changes were also prevented by concomitant treatment with antioxidants. 7 One study in HIV-infected subjects showed that daily supplementation with vitamin E, 800 IU, and vitamin C, 1000 mg, did result in a significant decrease in lipid oxidative markers and produced a trend toward a reduction in viral load. 8
We conducted this pilot trial of antioxidants to explore the possible contribution of oxidative stress to lipoatrophy or sustained hyperlactatemia. The study was approved by the Institutional Review Board of the University Hospitals of Cleveland; all subjects gave written informed consent. HIV-infected subjects from the University Hospitals of Cleveland John T. Carey Special Immunology Unit were approached to participate if they had either lipoatrophy or sustained hyperlactatemia and were receiving stable NRTI-containing therapy for at least 12 months. Lipoatrophy was defined as patients' self-report of decrease in fat in at least 2 of the following areas: face, arms, legs, or buttocks. This had to be confirmed by the investigator and by an experienced registered dietician. Sustained hyperlactatemia was defined as reproducible elevated lactate levels of >2x the upper limit of normal (>4.8 m M). Fasting venous lactate levels were collected following ACTG guidelines, without the use of tourniquet, fist clenching, and with immediate processing by the local laboratory. 9 No specific dietary advice was provided to the subjects. Patients were continued on their current antiretrovirals while antioxidants were added. The primary study end point was a change in peripheral fat, as assessed by the subject, the clinician, and by anthropometric measurements. Changes in CD4, HIV-1 RNA, and metabolic indices were assessed at weeks 0, 4, 8, 16, and 24. In addition, a registered dietician performed serial anthropometrics and bioelectrical impedance measurements of fat and lean body mass. Differences between baseline and on-treatment values were analyzed using Student t test; a 2-sided P < 0.05 was considered significant.
Ten subjects (2 women) aged 43 years (range 35-50) were enrolled into this pilot trial. All subjects were judged to be adherent to their antiretroviral regimen. Nine subjects had lipoatrophy at baseline. The only subject enrolled with no lipoatrophy had sustained hyperlactatemia for 4 months prior to study entry.
All subjects received open label treatment with vitamin E at 800 IU/d, vitamin C at 1000 mg/d, and N-acetyl cysteine (NAC) at 600 mg twice daily. The NAC was compounded into a capsule formulation by a local registered pharmacist, because only a foul-smelling liquid formulation is available in the United States. All subjects were receiving a stable NRTI-containing regimen for a median duration of 34 ± 16 months prior to study entry. The NRTIs at study entry were stavudine/lamivudine (4), zidovudine/lamivudine (3), didanosine/stavudine (1), didanosine/lamivudine (1), and abacavir/lamivudine (1). Eight were also receiving 1-2 protease inhibitor(s); the other 2 were receiving efavirenz. The duration of antiretroviral therapy prior to study entry was 76 ± 34 months. Baseline CD4 was 627 ± 316 cells/mm3. Nine subjects had HIV-1 RNA of <400 copies/mL at study entry.
Table 1 summarizes the baseline data and the changes after 24 weeks of study drugs. Lactate levels were normal in the 9 subjects with lipoatrophy. In the 1 subject with sustained asymptomatic hyperlactatemia, lactate was 4.92 at study entry. All subjects tolerated study drugs without adverse events. One subject was lost to follow-up at week 8 and therefore was excluded from the analysis. The 1 subject with hyperlactatemia decided to enroll in a structured treatment interruption study and discontinued his antiretrovirals at week 20. His lactate was 3.73 at week 16 and 1.3 at week 24. Because of the on-study discontinuation of antiretrovirals as well as the lack of lipoatrophy, he was excluded from the final analysis.
Neither clinician, registered dietician, nor patients noticed any improvement of the study subjects' peripheral fat. There was no modification of exercise, diet, lipid-lowering drugs, or insulin-sensitizing agents throughout the study period. Fasting (≥12 hours) triglycerides and total and high-density lipoprotein cholesterol levels did not change significantly. Fasting low-density lipoprotein cholesterol (calculated) trended down from 124 ± 48 mg/dL at baseline to 102 ± 32 mg/dL at week 24 (P = 0.06).
Standardized anthropometrics revealed no change in circumferences or skinfold thickness; waist-to-hip ratio decreased from 0.94 ± 0.06 at baseline to 0.92 ± 0.05 at week 24 (P = 0.05). Fasting glucose increased significantly from a median of 84 ± 11 mg/dL at baseline to 110 ± 21 mg/dL at week 24 (P = 0.017). Fasting insulin trended upward from 14.2 ± 15.8 IU/mL at baseline to 54 ± 76 IU/mL at week 24 (P = 0.1). Homeostatic model assessment of insulin resistance (HOMA-IR), a widely used index of insulin sensitivity, significantly increased from 2.75 ± 2.69 at study entry to 7.20 ± 5.77 at week 24 (P = 0.03). Similarly, C peptide trended upward from 3.7 ± 2.4 ng/mL at baseline to 7.7 ± 6 ng/mL at week 24 (P = 0.093). Serum lactate and transaminases remained unchanged.
Lipoatrophy is a distressing long-term complication associated with decreased quality of life and disincentive for adherence to antiretrovirals. 10,11 To date several strategies have been unsuccessful in reversing this condition; a partially successful one has been the replacement of stavudine with abacavir. 12-14 However, subjects may experience lipoatrophy while on abacavir, or in some instances, this strategy may not be suitable because of prior antiretroviral experience. This suggests the need to investigate strategies that do not require switches in antiretroviral therapy.
There is a rationale for testing of antioxidants in lipoatrophy and hyperlactatemia. The choice of the dose and type of antioxidants for this study was based on studies showing success in preventing NRTI-induced mitochondrial toxicity 6,7 and in decreasing lipid oxidation markers. 8 The antioxidants used in this pilot trial were well tolerated, except for an increase in fasting glucose and in HOMA-IR, a widely used index of insulin resistance, which accounts for both fasting insulin and glucose levels and is known to correlate well with clamp techniques. These findings are of concern and suggest increasing insulin resistance. Alternatively, this could represent the natural history of insulin resistance in lipoatrophy. Favorable trends toward lower low-density lipoprotein cholesterol and waist-to-hip ratios are encouraging. We acknowledge the limitations of this study, mainly the small sample size and the lack of sensitive fat imaging, such as CT scan or MRI. Therefore this study was unable to detect small changes in peripheral fat. Controlled studies are required to evaluate directly the effects of these agents on lipid and glucose metabolism.
REFERENCES
1. Brinkman K, Smeitink JA, Romijn JA, et al. Mitochondrial toxicity induced by nucleoside-analogue reverse-transcriptase inhibitors is a key factor in the pathogenesis of antiretroviral-therapy-related lipodystrophy. Lancet. 1999, 354:1112-1115.
2. Saint-Marc T, Partisani M, Poizot-Martin I, et al. A syndrome of peripheral fat wasting (lipodystrophy) in patients receiving long-term nucleoside analogue therapy. AIDS. 1999, 13:1659-1667.
3. Mallal SA, John M, Moore CB, et al. Contribution of nucleoside analogue reverse transcriptase inhibitors to subcutaneous fat wasting in patients with HIV infection. AIDS. 2000, 14:1309-1316.
4. Carr A, Miller J, Law M, et al. A syndrome of lipoatrophy, lactic academia and liver dysfunction associated with HIV nucleoside analogue therapy: contribution to protease inhibitor-related lipodystrophy syndrome. AIDS. 2000; 14:F25-F32.
5. Hayakawa M, Ogawa T, Sugiyama S, et al. Massive conversion of guanosine to 8-hydroxy-guanosine in mouse liver mitochondrial DNA by administration of azidothymidine. Biochem Biophys Commun. 1991; 29:606-614.
6. de la Asuncion JG, del Olmo ML, Sastre J, et al. AZT treatment induces molecular and ultrastructural oxidative damage to muscle mitochondria: prevention by antioxidant vitamins. J Clin Invest. 1998; 102:4-9.
7. Paulik M, Lancaster M, Croom D, et al. Anti-oxidants rescue NRTI-induced metabolic changes in AKR/J mice. Antivir Ther. 2000; 5(suppl 5):6-7.
8. Allard JP, Aghdassi E, Chau J, et al. Effects of vitamin E and C supplementation on oxidative stress and viral load in HIV-infected subjects. AIDS. 1998; 12:1653-1659.
9. AACTG lactic acidosis guidelines. Available at http://aactg.s-3.com/members/download/other/metabolic/lacacid.doc . Accessed July 2, 2003.
10. Duran S, Saves M, Spire B, and the APROCO Study Group. Failure to maintain long-term adherence to highly active antiretroviral therapy: the role of lipodystrophy. AIDS. 2001; 15:2441-2444.
11. Dukersa NH, Stoltea IG, Albrechta N, et al. The impact of experiencing lipodystrophy on the sexual behavior and well-being among HIV-infected homosexual men. AIDS. 2001; 15:812-813.
12. McComsey G, Ward D, Hessenthaler S, et al. CT scan findings at 48 weeks confirm further regression of lipoatrophy following the substitution of stavudine (d4T) with either abacavir (ABC) or zidovudine (ZDV). Paper presented at: 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy; September 27-30, 2002; San Diego, CA. Abstract H-1929.
13. Carr A, Smith D, Workman C, et al. Switching stavudine or zidovudine to abacavir for HIV lipoatrophy: a randomized, controlled, open-label, multicentered, 24-week study. Paper presented at: 9th Conference on Retroviruses and Opportunistic Infections; February 24-28, 2002; Seattle, WA.
14. John M, James I, McKinnon E, et al. A randomized, controlled, open-label study of revision of antiretroviral regimens containing stavudine (d4T) and/or a protease inhibitor (PI) to zidovudine (ZDV)/lamivudine (3TC)/abacavir (ABC) to prevent or reverse lipoatrophy: 48-week data. Paper presented at: 9th Conference on Retroviruses and Opportunistic Infections; February 24-28, 2002; Seattle, WA.
|
|
| |
| |
|
|
|